



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
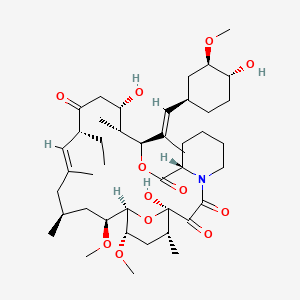

1. Changchuanmycin
2. Fk 520
3. Fk-520
4. Fr 900520
5. Fr-900520
6. Immunomycin
7. Tacrolimus Related Compound A
1. Immunomycin
2. 104987-12-4
3. Fk520
4. Fr 900520
5. Fk 520
6. L-683590
7. Auf4u5nsjk
8. Fk-520
9. L 683590
10. Chembl8597
11. Chebi:29582
12. Fr-900520
13. 11011-38-4
14. Ascomycin From Streptomyces Hygroscopicus Var. Ascomyceticus
15. Changchuanmycin
16. Ascomycin(fk 520)
17. (3s,4r,5s,8r,9e,12s,14s,15r,16s,18r,19r,22r,26as)-8-ethyl-5,19-dihydroxy-3-{(1e)-1-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-14,16-dimethoxy-4,10,12,18-tetramethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-3h-15,19-epoxypyrido[2,1-c][1,4]oxazacyclotricosine-1,7,20,21(4h,23h)-tetrone
18. C43h69no12
19. Unii-auf4u5nsjk
20. Tacrolimus Related Compound A
21. Ascomycin Solution
22. Fr 520
23. Mfcd06198665
24. Nsc 106410
25. Nsc-106410
26. Ascomycin (fk520)
27. Schembl25512
28. Bspbio_001318
29. L 683,590
30. L-683,590
31. Chebi:94818
32. Dtxsid80894126
33. Hms1361b20
34. Hms1791b20
35. Hms1989b20
36. Hms3402b20
37. Ex-a1796
38. Bdbm50068939
39. Fr-520
40. S7411
41. Akos015916691
42. Zinc169289416
43. Ccg-270483
44. Cs-1516
45. Idi1_033788
46. Ncgc00163418-01
47. Ncgc00163418-02
48. 15,19-epoxy-3h-pyrido(2,1-c)(1,4)oxaazacyclotricosine-1,7,20,21(4h,23h)-tetrone, 8-ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-(2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl)-14,16-dimethoxy-4,10,12,18-tetram
49. Hy-13557
50. Ascomycin (fk 520, Fr 900520, Imm
51. Tacrolimus Related Compound A [usp-rs]
52. 011a384
53. Q4803998
54. Tacrolimus Related Compound A [usp Impurity]
55. Brd-k88998544-001-02-4
56. Brd-k99369265-001-01-4
57. Tacrolimus Impurity, Ascomycin- [usp Impurity]
58. Tacrolimus Monohydrate Impurity A [ep Impurity]
59. (1r,9s,12s,13r,14s,17r,18e,21s,23s,24r,25s,27r)-17-ethyl-1,14-dihydroxy-12-[(1e)-1-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.0?,?]octacos-18-ene-2,3,10,16-tetrone
60. (1r,9s,12s,13r,14s,17r,18e,21s,23s,24r,25s,27r)-17-ethyl-1,14-dihydroxy-12-[(e)-1-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-azatricyclo[22.3.1.04,9]octacos-18-ene-2,3,10,16-tetrone
61. (3s,4r,5s,8r,9e,12s,14s,15r,16s,18r ,19r,26as)-8-ethyl-5,6,8,11,12,13,14,15,16,17,18,1 9,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(1e )-2-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetrameth Yl-15,19-epoxy-3h-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4h,23h)tetrone
62. (3s,4r,5s,8r,9e,12s,14s,15r,16s,18r,19r,26as)-8-ethyl-5,19-dihydroxy-3-{(1e)-1-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl}-14,16-dimethoxy-4,10,12,18-tetramethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-3h-15,19-epoxypyrido[2,1-c][1,4]oxazacyclotricosine-1,7,20,21(4h,23h)-tetrone
63. (3s,4r,5s,8r,9e,12s,14s,15r,16s,18r,19r,26as)-8-ethyl-5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-3-[(1e)-2-[(1r,3r,4r)-4-hydroxy-3-methoxycyclohexyl]-1-methylethenyl]-14,16-dimethoxy-4,10,12,18-tetramethyl-15,19-epoxy-3h-pyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(4h,23h)tetrone
64. (ascomycin)17-ethyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]octacos-18-ene-2,3,10,16-tetraone
65. (e)-(9s,12s,13r,14s,17r,21s,23s,24r,25s,27s)-17-ethyl-1,14-dihydroxy-12-[(e)-2-((1r,3r,4r)-4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]octacos-18-ene-2,3,10,16-tetraone
66. (e)-(s)-17-ethyl-1,14-dihydroxy-12-[(e)-2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]oct Acos-18-ene-2,3,10,16-tetraone
67. (e)-17-ethyl-1,14-dihydroxy-12-[(e)-2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]octacos-18-ene-2,3,10,16-tetraone
68. 135635-46-0
69. 15,19-epoxy-3h-pyrido(2,1-c)(1,4)oxaazacyclotricosine-1,7,20,21(4h,23h)-tetrone, 5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-5,19-dihydroxy-8-ethyl-3-(2-(4-hydroxy-3-methoxycyclohexyl)-1-methylethenyl)-14,16-dimethoxy-4,10,12,18-tetramethyl-, (3s-(3r*(e(1s*,3s*,4s*)),4s*,19s*,26ar*))-
70. 17-ethyl-1,14-dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-vinyl]-23,25-dimethoxy-13,19,21,27-tetramethyl-11,28-dioxa-4-aza-tricyclo[22.3.1.0*4,9*]octacos-18-ene-2,3,10,16-tetraone
| Molecular Weight | 792.0 g/mol |
|---|---|
| Molecular Formula | C43H69NO12 |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 6 |
| Exact Mass | 791.48197664 g/mol |
| Monoisotopic Mass | 791.48197664 g/mol |
| Topological Polar Surface Area | 178 Ų |
| Heavy Atom Count | 56 |
| Formal Charge | 0 |
| Complexity | 1430 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
Immunosuppressive Agents
Agents that suppress immune function by one of several mechanisms of action. Classical cytotoxic immunosuppressants act by inhibiting DNA synthesis. Others may act through activation of T-CELLS or by inhibiting the activation of HELPER CELLS. While immunosuppression has been brought about in the past primarily to prevent rejection of transplanted organs, new applications involving mediation of the effects of INTERLEUKINS and other CYTOKINES are emerging. (See all compounds classified as Immunosuppressive Agents.)


