



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
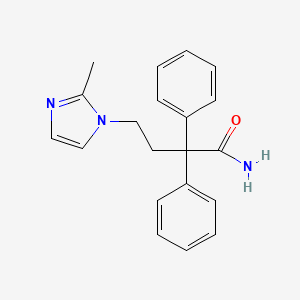

1. Krp 197
2. Krp-197
1. 170105-16-5
2. Uritos
3. Staybla
4. Krp-197
5. 4-(2-methyl-1h-imidazol-1-yl)-2,2-diphenylbutanamide
6. Imidafenacin [inn]
7. 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutanamide
8. Ono-8025
9. Xjr8y07ljo
10. 4-(2-methylimidazol-1-yl)-2,2-diphenylbutanamide
11. Krp-197;ono-8025
12. Krp 197
13. Ono 8025
14. Unii-xjr8y07ljo
15. Staybla (tn)
16. Uritos (tn)
17. Imidafenacin (jan/inn)
18. Imidafenacin [mi]
19. Imidafenacin [jan]
20. Imidafenacin [mart.]
21. Chembl53366
22. Imidafenacin [who-dd]
23. Schembl929680
24. Zinc7368
25. Dtxsid00870104
26. Chebi:134720
27. Hms3886c04
28. Bcp10054
29. Ex-a4417
30. Hy-b0662
31. Mfcd09833703
32. S5385
33. Akos030526649
34. Am84591
35. Ccg-267694
36. Cs-3681
37. Db09262
38. Ncgc00183110-01
39. Ncgc00183110-02
40. As-47648
41. Db-119256
42. Imidafenacin Hydrochloride, >=95% (hplc)
43. Ft-0670290
44. D06273
45. F14841
46. 105i165
47. A882053
48. L001601
49. J-521484
50. Q6003989
51. 1h-imidazole-1-butanamide, 2-methyl-alpha,alpha-diphenyl-
52. 4-(2-methyl-1-imidazolyl)-2,2-diphenylbutanamide Hydrochloride
53. 2-methyl-
54. A,
55. A-diphenyl-1h-imidazole-1-butanamide Hydrochloride
56. 4-(2-methyl-1h-imidazol-1-yl)-2,2-diphenylbutanamide, Aldrichcpr
| Molecular Weight | 319.4 g/mol |
|---|---|
| Molecular Formula | C20H21N3O |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 6 |
| Exact Mass | 319.168462302 g/mol |
| Monoisotopic Mass | 319.168462302 g/mol |
| Topological Polar Surface Area | 60.9 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 395 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used in the treatment of overactive bladder.
FDA Label
Imidafenacin is an antimuscarinic agent which acts to reduce the frequency of urination in patients with overactive bladder.
Absorption
The absolute oral bioavailability is 57.8%. Tmax is 1-3 h after administration.
Route of Elimination
10% is excreted in the urine as the parent compound. Most is eliminated by metabolism thought to be mediated by CYP3A4 and UGT1A4.
Volume of Distribution
The estimated volume of distribution is 43.9 L.
Clearance
The estimated clearance is 21.2 L/h.
Thought to be metabolized v by CYP3A4 and UGT1A4. No active metabolites have been observed.
The half life of elimination is 3 h.
Imidafenacin binds to and antagonizes muscarinic M1 and M3 receptors with high affinity. It also antagonizes muscarinic M2 receptors but with lower affinity. M3 receptors stimulate contraction of the detrusor muscle in the bladder via release of calcium from the sarcoplasmic reticulum. M2 receptors are also present in the detrusor muscle but serve to inhibit adenylate cyclase which reduces the relaxation mediated by adrenergic receptors. Finally, M1 receptors are present on the parasympathetic neurons which release acetylcholine in the bladder. They act as an autocrine positive feedback loop and further increase release of acetylcholine. Antagonism of these receptors by imidafenacin prevents contraction of the bladder's detrusor muscle, prevents inhibition of the relation produced by sympathetic tone, and reduces acetylcholine release. Together these reduce the frequency of urination.


