


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
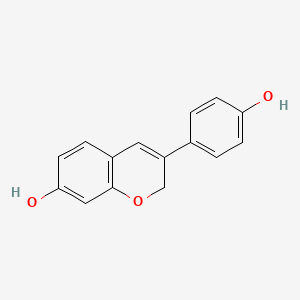

1. 7-hydroxy-3-hydroxyphenyl-1h-benzopyran
2. Phenoxodiol
1. Phenoxodiol
2. Dehydroequol
3. 81267-65-4
4. Haginin E
5. 3-(4-hydroxyphenyl)-2h-chromen-7-ol
6. 3-(4-hydroxyphenyl)-2h-1-benzopyran-7-ol
7. Nv-06
8. 7,4'-dihydroxyisoflav-3-ene
9. 2h-1-benzopyran-7-ol, 3-(4-hydroxyphenyl)-
10. Idronoxil;dehydroequol;haginin E
11. 995ft1w541
12. Nv 06
13. Idronoxil [usan]
14. Ccris 8949
15. Idronoxil (usan/inn)
16. Idronoxil [usan:inn]
17. 7-hydroxy-3-hydroxyphenyl-1h-benzopyran
18. Haganin E
19. Unii-995ft1w541
20. Idronoxil [inn]
21. Phenoxodiol (haginin E)
22. Dehydroequol [mi]
23. Isoflav-3-ene4',7-diol
24. Idronoxil [who-dd]
25. Isoflav-3-ene-4',7-diol
26. Mls006010720
27. Schembl149612
28. 4',7-dihydroxyisoflav-3-ene
29. Chembl1957038
30. Phenoxodiol, >=98% (hplc)
31. Dtxsid50231029
32. ...7,4?-dihydroxyisoflav-3-ene
33. Nox-66 Component Idronoxil
34. Zinc1491943
35. Bdbm50419932
36. S9634
37. Akos015918005
38. Db04915
39. 3-(4-hydroxy-phenyl)-2h-chromen-7-ol
40. Ncgc00346822-01
41. Ncgc00346822-02
42. Hy-13721
43. Smr004701684
44. Xd161580
45. Xd161694
46. Db-012327
47. Cs-0007751
48. Ft-0602222
49. Nox66 Suppository Component Idronoxil
50. D04498
51. Idronoxil Component Of Nox66 Suppository
52. 267p654
53. A853212
54. Q27095562
55. (+/-)-cis-3-(4-hydroxyphenyl)-4-(4-methoxyphenyl)-3,4-dihydro-2h-cromen-7-ol
| Molecular Weight | 240.25 g/mol |
|---|---|
| Molecular Formula | C15H12O3 |
| XLogP3 | 2.7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 240.078644241 g/mol |
| Monoisotopic Mass | 240.078644241 g/mol |
| Topological Polar Surface Area | 49.7 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 318 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Intended for the treatment of various forms of cancer.
Phenoxodiol inhibits proliferation of many cancer cell lines and induces apoptosis by disrupting FLICE-inhibitory protein, FLIP, expression and by caspase-dependent and -independent degradation of the X-linked inhibitor of apoptosis, XIAP. In addition, phenoxodiol sensitizes drug-resistant tumour cells to anticancer drugs including paclitaxel, carboplatin and gemcitabine.
The antiproliferative effects of phenoxodiol are associated with inhibition of plasma membrane electron transport in tumour cell lines and primary immune cells. Results from one study (PMID: 17904534) indicate that plasma membrane electron transport (PMET) may be a primary target for phenoxodiol in tumour cells and in activated T cells.


