



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada
0

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
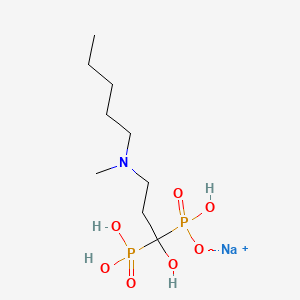

1. (1-hydroxy-3-(methylpentylamino)propylidene)bisphosphonate
2. 1-hydroxy-3-(methylpentylamino)propylidenebisphosphonate
3. Bm 21.0955
4. Bm 210955
5. Bm-21.0955
6. Bm-210955
7. Bm21.0955
8. Bm210955
9. Bondronat
10. Boniva
11. Bonviva
12. Ibandronate
13. Ibandronate Sodium Anhydrous
14. Ibandronic Acid
15. Ibandronic Acid, Sodium Salt, Monohydrate
16. Rpr 102289a
17. Rpr-102289a
18. Rpr102289a
1. 138844-81-2
2. Bondronat
3. Ibandronate Sodium Salt
4. Boniva
5. Ibandronate (sodium)
6. Sodium Ibandronate
7. Ibandronate Sodium Anhydrous
8. Bonviva
9. 23y0b94e49
10. (1-hydroxy-3-(methylpentylamino)propylidene)bisphosphonic Acid Sodium
11. Sodium;hydroxy-[1-hydroxy-3-[methyl(pentyl)amino]-1-phosphonopropyl]phosphinate
12. Phosphonic Acid, (1-hydroxy-3-(methylpentylamino)propylidene)bis-, Monosodium Salt
13. Bm 21.0955na
14. Ncgc00167428-01
15. Bm-21.0955
16. Unii-23y0b94e49
17. Dsstox_cid_26618
18. Dsstox_rid_81770
19. Dsstox_gsid_46618
20. Schembl1030768
21. Chembl1201008
22. Dtxsid8046618
23. Hy-b0515b
24. Hms3263m08
25. Hms3714g19
26. Sodium Ibandronate Anhydrous
27. Bcp22744
28. Ibandronate Sodium [who-dd]
29. Tox21_112432
30. Tox21_501103
31. Mfcd07197214
32. Akos026750153
33. Bcp9000767
34. Ccg-220679
35. Ccg-222407
36. Lp01103
37. Ibandronic Acid Sodium Salt [mi]
38. Ncgc00261788-01
39. Cas-138844-81-2
40. Ft-0670252
41. S0877
42. H11437
43. Ibandronate Sodium Salt, >=97% (nmr), Solid
44. J-007183
45. Q27253790
46. (1-hydroxy-3-(methyl(pentyl)amino)propane-1,1-diyl)diphosphonic Acid Monosodium Salt
| Molecular Weight | 341.21 g/mol |
|---|---|
| Molecular Formula | C9H22NNaO7P2 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 9 |
| Exact Mass | 341.07692032 g/mol |
| Monoisotopic Mass | 341.07692032 g/mol |
| Topological Polar Surface Area | 141 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 377 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Boniva |
| PubMed Health | Ibandronate |
| Drug Classes | Calcium Regulator |
| Drug Label | BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, mon... |
| Active Ingredient | Ibandronate sodium |
| Dosage Form | Injectable; Tablet |
| Route | Intravenous; Oral |
| Strength | eq 3mg base/3ml; eq 150mg base |
| Market Status | Prescription |
| Company | Hoffmann La Roche; Roche |
| 2 of 4 | |
|---|---|
| Drug Name | Ibandronate sodium |
| Drug Label | BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, mon... |
| Active Ingredient | Ibandronate sodium |
| Dosage Form | Tablet; Injectable |
| Route | injection; oral; Oral; Intravenous |
| Strength | 2.5mg; 1mg; eq 150mg base; eq 3mg base/3ml |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Teva Parenteral; Apotex; Sun Pharm Inds; Watson Labs; Emcure Pharms; Mutual Pharm; Cobalt Labs; Sagent Pharms; Dr Reddys Labs; Agila Speclts; Orchid Hlthcare |
| 3 of 4 | |
|---|---|
| Drug Name | Boniva |
| PubMed Health | Ibandronate |
| Drug Classes | Calcium Regulator |
| Drug Label | BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, mon... |
| Active Ingredient | Ibandronate sodium |
| Dosage Form | Injectable; Tablet |
| Route | Intravenous; Oral |
| Strength | eq 3mg base/3ml; eq 150mg base |
| Market Status | Prescription |
| Company | Hoffmann La Roche; Roche |
| 4 of 4 | |
|---|---|
| Drug Name | Ibandronate sodium |
| Drug Label | BONIVA (ibandronate sodium) is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid, monosodium salt, mon... |
| Active Ingredient | Ibandronate sodium |
| Dosage Form | Tablet; Injectable |
| Route | injection; oral; Oral; Intravenous |
| Strength | 2.5mg; 1mg; eq 150mg base; eq 3mg base/3ml |
| Market Status | Tentative Approval; Prescription |
| Company | Mylan Pharms; Teva Parenteral; Apotex; Sun Pharm Inds; Watson Labs; Emcure Pharms; Mutual Pharm; Cobalt Labs; Sagent Pharms; Dr Reddys Labs; Agila Speclts; Orchid Hlthcare |
Treatment of osteoporosis in postmenopausal women at increased risk of fracture (see section 5. 1). A reduction in the risk of vertebral fractures has been demonstrated, efficacy on femoral neck fractures has not been established.
Bondronat is indicated for:
- prevention of skeletal events (pathological fractures, bone complications requiring radiotherapy or surgery) in patients with breast cancer and bone metastases;
- treatment of tumour-induced hypercalcaemia with or without metastases.
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
M05BA06
M05BA06








