
Synopsis
Synopsis
0
JDMF
0
VMF
0
Australia
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
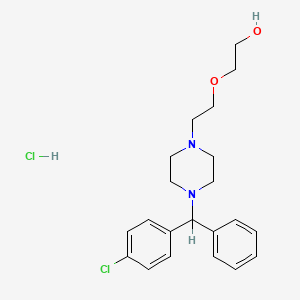

1. 2-(2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)ethanol
2. Atarax
3. Durrax
4. Hydroxyzine
5. Hydroxyzine Dihydrochloride
6. Hydroxyzine Pamoate
7. Orgatrax
8. Pamoate, Hydroxyzine
9. Vistaril
1. Hydroxyzine Hcl
2. 1244-76-4
3. Hydroxyzine Monohydrochloride
4. Ca67jf5q4w
5. Mls000028602
6. Smr000058737
7. 2-(2-(4-((4-chlorophenyl)phenylmethyl)piperazin-1-yl)ethoxy)ethanol Hydrochloride
8. 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]ethanol;hydrochloride
9. 2-[2-[4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]ethanol Hydrochloride
10. Einecs 214-989-3
11. Unii-ca67jf5q4w
12. Hydroxyzin Hydrochloride
13. Opera_id_1515
14. 1-(p-chlorobenzhydryl)-4-(2-(2-hydroxyethoxy)ethyl)diethylenediamine Hydrochloride
15. Dsstox_cid_27530
16. Dsstox_rid_82400
17. Dsstox_gsid_47530
18. Schembl41448
19. Mls002222192
20. Chembl1201007
21. Dtxsid5047530
22. Tox21_302563
23. Ethanol, 2-(2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-, Monohydrochloride
24. Ethanol, 2-(2-(4-(p-chloro-alpha-phenylbenzyl)-1-piperazinyl)ethoxy)-, Hydrochloride
25. Ethanol, 2-(2-(4-(p-chloro-alpha-phenylbenzyl)-1-piperazinyl)ethoxy)-, Monohydrochloride
26. Ncgc00180974-01
27. Ncgc00256629-01
28. 14729-22-7
29. Cas-1244-76-4
30. Q27275377
31. 2-[2-[4-[(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethoxy]ethanol;hydron;chloride
32. 2-[2-[4-[(4-chlorophenyl)phenylmethyl]piperazin-1-yl]ethoxy]ethanolhydrochloride
33. Ethanol, 2-(2-(4-((4-chlorophenyl)phenylmethyl)-1-piperazinyl)ethoxy)-, Hydrochloride (1:1)
34. Ethanol, 2-(2-(4-(p-chloro-.alpha.-phenylbenzyl)-1-piperazinyl)ethoxy)-, Monohydrochloride
| Molecular Weight | 411.4 g/mol |
|---|---|
| Molecular Formula | C21H28Cl2N2O2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 8 |
| Exact Mass | 410.1527835 g/mol |
| Monoisotopic Mass | 410.1527835 g/mol |
| Topological Polar Surface Area | 35.9 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 376 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antipruritics
Agents, usually topical, that relieve itching (pruritus). (See all compounds classified as Antipruritics.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)
 Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
About the Company : Cosma S.p.A., part of the CFM Group with AMSA and Clarochem Ireland, is the group’s largest manufacturing site in Bergamo, Italy. With 130,000L glass-lined reaction volume and ov...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for pharmaceutical and biotech industries. LGM also operates as a full-service CDMO, offering formulat...
About the Company : Jai Radhe Sales, founded in 1999, is a global distributor specializing in high-quality pharmaceutical ingredients from India. It offers complete sourcing solutions, technical and r...
 Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
Malladi is a leader in Ephedrine, Pseudoephedrine Salts & Phenylephrine HCl // USFDA, EDQM, ANSM, KFDA, and TGA inspected.
About the Company : Malladi Drugs & Pharmaceuticals Ltd, founded in 1980 by Mr. M L N Sastry, is a leader in manufacturing ephedrine and pseudoephedrine salts. It is now the top API manufacturer in th...
About the Company : Since 1962, MOEHS has produced high-quality Active Pharmaceutical Ingredients (APIs) for the global market. With decades of technical expertise, Moehs Group delivers pharmaceutical...
About the Company : HRV Pharma is a global manufacturer, seller, and exporter of APIs, intermediates, pellets, food-grade chemicals, food additives, and food ingredients. The company provides sourcing...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
About the Company : Our endeavours also lie in the import, and domestic sourcing of raw materials from reliable vendors of the market. We have an excellent infrastructure with world class cGMP facilit...
 Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
Willow Birch Pharma delivers trusted, high-quality APIs nationwide with unmatched service, compliance, and competitive value.
About the Company : Willow Birch Pharma, Inc. is a premier supplier of bulk APIs to North American Compounding Pharmacies, sourcing from FDA registered and GMP manufacturers. With licenses in all 50 s...
About the Company : Fleming Laboratories Limited is in the business of manufacturing and supply of high-quality generic Active Pharmaceutical Ingredients (APIs) to the global Pharmaceutical Industry. ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
77
PharmaCompass offers a list of Hydroxyzine Dihydrochloride API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Hydroxyzine Dihydrochloride manufacturer or Hydroxyzine Dihydrochloride supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Hydroxyzine Dihydrochloride manufacturer or Hydroxyzine Dihydrochloride supplier.
PharmaCompass also assists you with knowing the Hydroxyzine Dihydrochloride API Price utilized in the formulation of products. Hydroxyzine Dihydrochloride API Price is not always fixed or binding as the Hydroxyzine Dihydrochloride Price is obtained through a variety of data sources. The Hydroxyzine Dihydrochloride Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Hydroxyzine HCl manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Hydroxyzine HCl, including repackagers and relabelers. The FDA regulates Hydroxyzine HCl manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Hydroxyzine HCl API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Hydroxyzine HCl manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Hydroxyzine HCl supplier is an individual or a company that provides Hydroxyzine HCl active pharmaceutical ingredient (API) or Hydroxyzine HCl finished formulations upon request. The Hydroxyzine HCl suppliers may include Hydroxyzine HCl API manufacturers, exporters, distributors and traders.
click here to find a list of Hydroxyzine HCl suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Hydroxyzine HCl DMF (Drug Master File) is a document detailing the whole manufacturing process of Hydroxyzine HCl active pharmaceutical ingredient (API) in detail. Different forms of Hydroxyzine HCl DMFs exist exist since differing nations have different regulations, such as Hydroxyzine HCl USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Hydroxyzine HCl DMF submitted to regulatory agencies in the US is known as a USDMF. Hydroxyzine HCl USDMF includes data on Hydroxyzine HCl's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Hydroxyzine HCl USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Hydroxyzine HCl suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Hydroxyzine HCl Drug Master File in Korea (Hydroxyzine HCl KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Hydroxyzine HCl. The MFDS reviews the Hydroxyzine HCl KDMF as part of the drug registration process and uses the information provided in the Hydroxyzine HCl KDMF to evaluate the safety and efficacy of the drug.
After submitting a Hydroxyzine HCl KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Hydroxyzine HCl API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Hydroxyzine HCl suppliers with KDMF on PharmaCompass.
A Hydroxyzine HCl CEP of the European Pharmacopoeia monograph is often referred to as a Hydroxyzine HCl Certificate of Suitability (COS). The purpose of a Hydroxyzine HCl CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Hydroxyzine HCl EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Hydroxyzine HCl to their clients by showing that a Hydroxyzine HCl CEP has been issued for it. The manufacturer submits a Hydroxyzine HCl CEP (COS) as part of the market authorization procedure, and it takes on the role of a Hydroxyzine HCl CEP holder for the record. Additionally, the data presented in the Hydroxyzine HCl CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Hydroxyzine HCl DMF.
A Hydroxyzine HCl CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Hydroxyzine HCl CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Hydroxyzine HCl suppliers with CEP (COS) on PharmaCompass.
A Hydroxyzine HCl written confirmation (Hydroxyzine HCl WC) is an official document issued by a regulatory agency to a Hydroxyzine HCl manufacturer, verifying that the manufacturing facility of a Hydroxyzine HCl active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Hydroxyzine HCl APIs or Hydroxyzine HCl finished pharmaceutical products to another nation, regulatory agencies frequently require a Hydroxyzine HCl WC (written confirmation) as part of the regulatory process.
click here to find a list of Hydroxyzine HCl suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Hydroxyzine HCl as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Hydroxyzine HCl API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Hydroxyzine HCl as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Hydroxyzine HCl and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Hydroxyzine HCl NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Hydroxyzine HCl suppliers with NDC on PharmaCompass.
Hydroxyzine HCl Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Hydroxyzine HCl GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Hydroxyzine HCl GMP manufacturer or Hydroxyzine HCl GMP API supplier for your needs.
A Hydroxyzine HCl CoA (Certificate of Analysis) is a formal document that attests to Hydroxyzine HCl's compliance with Hydroxyzine HCl specifications and serves as a tool for batch-level quality control.
Hydroxyzine HCl CoA mostly includes findings from lab analyses of a specific batch. For each Hydroxyzine HCl CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Hydroxyzine HCl may be tested according to a variety of international standards, such as European Pharmacopoeia (Hydroxyzine HCl EP), Hydroxyzine HCl JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Hydroxyzine HCl USP).

