



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Aldiamed
2. Hydroxyethylcellulose
3. Hydroxyethylcellulose, Sodium Salt
4. Hydroxyl Ethyl Cellulose
5. Lacrigel
6. Minims Artificial Tears
7. Natrosol 250
1. Hetastarch
2. 9004-62-0
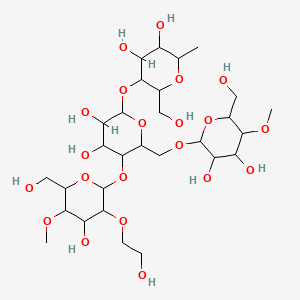
3. 5-[6-[[3,4-dihydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxymethyl]-3,4-dihydroxy-5-[4-hydroxy-3-(2-hydroxyethoxy)-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxyoxan-2-yl]oxy-6-(hydroxymethyl)-2-methyloxane-3,4-diol
4. 2-hydroxyethyl Cellulose
5. Hydroxyethyl-cellulose
6. Schembl23306563
7. Dtxsid60873934
8. Ft-0627136
9. H11622
10. 2-hydroxyethyl Cellulose; Cellulose Hydroxyethyl Ether
11. 2-o-(2-hydroxyethyl)-4-o-methylhexopyranosyl-(1->4)-[4-o-methylhexopyranosyl-(1->6)]hexopyranosyl-(1->5)-2,6-anhydro-1-deoxyheptitol
| Molecular Weight | 736.7 g/mol |
|---|---|
| Molecular Formula | C29H52O21 |
| XLogP3 | -7.7 |
| Hydrogen Bond Donor Count | 11 |
| Hydrogen Bond Acceptor Count | 21 |
| Rotatable Bond Count | 15 |
| Exact Mass | 736.30010866 g/mol |
| Monoisotopic Mass | 736.30010866 g/mol |
| Topological Polar Surface Area | 315 Ų |
| Heavy Atom Count | 50 |
| Formal Charge | 0 |
| Complexity | 999 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 20 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


