


API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
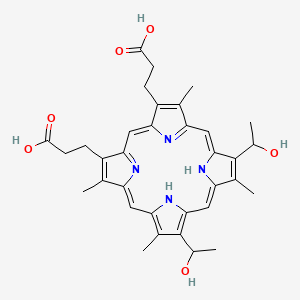

1. Haematoporphyrin Ix
2. Hematoporphyrins
3. Hemedonin
1. Haematoporphyrin
2. 14459-29-1
3. Hematoporphyrin Ix
4. Photodyn
5. Hematoporphyrin I
6. Hemoporfin
7. Nsc 59265
8. Nsc59265
9. 21h,23h-porphine-2,18-dipropanoic Acid, 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-
10. Nsc-59265
11. 3-[18-(2-carboxyethyl)-8,13-bis(1-hydroxyethyl)-3,7,12,17-tetramethyl-22,23-dihydroporphyrin-2-yl]propanoic Acid
12. Hp
13. Nsc 267084
14. Nsc-267084
15. 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-21h,23h-porphine-2,18-dipropanoic Acid
16. Hbt6m5h379
17. Chebi:36162
18. Hematoporphyrin_i
19. 2,18-porphinedipropionic Acid, 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-
20. 2,4-bis(1-hydroxyethyl)-1,3,5,8-tetramethyl-5,7-porphindipropionsaeure
21. 1,3,5,8-tetramethyl-2,4-bis(alpha-hydroxyethyl)prophine-6,7-dipropionic Acid
22. 7,12-bis(1-hydroxyethyl)-2,8,13,17-tetramethyl-21h,23h-porphin-2,18-dipropionsaeure
23. Hematoporphyrins
24. 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-2,18-porphinedipropionic Acid
25. 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethylporphyrin-2,18-dipropanoic Acid
26. Hp (van)
27. 2, 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-
28. Hamatoporphyrin
29. Photosan 3
30. 1,5,8-tetramethyl-2,4-bis(.alpha.-hydroxyethyl)prophine-6,7-dipropionic Acid
31. 21h,18-dipropanoic Acid, 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-
32. Wln: T D5 I5 N5-16-5 A D- I- N- Am D-n I-m N-nj Eyq F J K2vq O2vq Tyq U
33. Hpix
34. Einecs 238-450-7
35. Mfcd00005077
36. Haemaporphyrin
37. Brn 0078957
38. Spectrum_001204
39. Spectrum2_000436
40. Spectrum3_001110
41. Spectrum4_001942
42. Spectrum5_000763
43. Hematoporphyrin [mi]
44. Hemoporfin [who-dd]
45. Unii-hbt6m5h379
46. Schembl15669
47. Schembl15670
48. Bspbio_002820
49. Kbiogr_002346
50. Kbioss_001684
51. Spbio_000452
52. Chembl317840
53. Chembl403729
54. Schembl1649168
55. Hematoporphyrin [who-dd]
56. Kbio2_001684
57. Kbio2_004252
58. Kbio2_006820
59. Kbio3_002040
60. Dtxsid00864508
61. Dtxsid901030645
62. Hy-b0754
63. Nsc267084
64. Ccg-214649
65. Sdccgmls-0066934.p001
66. 2,18-porphinedipropionic Acid, 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl- (van)
67. Ncgc00185009-01
68. Ncgc00185009-02
69. As-78345
70. Hematoporphyrin Base Cas 14459-29-1
71. Ft-0626879
72. A808241
73. Sr-05000002762
74. J-007971
75. Sr-05000002762-1
76. 1,3,5,8-tetramethyl-2,4-bis( -hydroxyethyl)porphine-6,7-dipropionate
77. 1,3,5,8-tetramethyl-2,4-bis( -hydroxyethyl)porphine-6,7-dipropionic Acid
78. 1,3,5,8-tetramethyl-2,4-bis(a-hydroxyethyl)porphine-6,7-dipropionate
79. 1,3,5,8-tetramethyl-2,4-bis(a-hydroxyethyl)porphine-6,7-dipropionic Acid
80. 1,3,5,8-tetramethyl-2,4-bis(alpha-hydroxyethyl)prophine-6,7-dipropionate
81. 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-2,18-porphinedipropionate
82. 1,3,5,8-tetramethyl-2,4-bis(.alpha.-hydroxyethyl)prophine-6,7-dipropionic Acid
83. 3,3'-[7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethylporphyrin-2,18-diyl]dipropanoic Acid
84. 8,13-bis(1-hydroxyethyl)-3,7,12,17-tetramethyl-21h,23h-porphyrin-2,18-dipropionic Acid
85. 136752-88-0
86. 21h,23h-porphine-2,18-dipropanoic Acid, 7(or 12)-(1-hydroxyethyl)-12(or 7)-(1-methoxyethyl)-3,8,13,17-tetramethyl-
87. 3-[(1z,4z,9z,15z)-18-(2-carboxyethyl)-8,13-bis(1-hydroxyethyl)-3,7,12,17-tetramethyl-21,23-dihydroporphyrin-2-yl]propanoic Acid
88. Photodyn And 8,13-bis(1-hydroxyethyl)-3,7,12,17-tetramethyl-21h,23h-porphine-2,18-dipropionic Acid
| Molecular Weight | 598.7 g/mol |
|---|---|
| Molecular Formula | C34H38N4O6 |
| XLogP3 | 2.1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 8 |
| Exact Mass | 598.27913494 g/mol |
| Monoisotopic Mass | 598.27913494 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 44 |
| Formal Charge | 0 |
| Complexity | 1020 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)


