
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
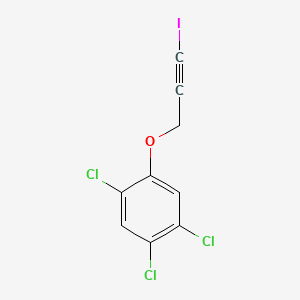

1. 3-iodo-2-propynyl 2,4,5-trichlorophenyl Ether
2. Halotex
3. Mycilan
1. 777-11-7
2. Halotex
3. Mycanden
4. Mycilan
5. Polik
6. M-1028 (meiji)
7. 1,2,4-trichloro-5-(3-iodoprop-2-ynoxy)benzene
8. M 1028
9. 3-iodo-2-propynyl 2,4,5-trichlorophenyl Ether
10. 2,4,5-trichlorophenyl Iodopropargyl Ether
11. Nsc-100071
12. Ether, 3-iodo-2-propynyl 2,4,5-trichlorophenyl
13. Haloprogina
14. Haloprogine
15. Aiu7053owl
16. Chembl1289
17. Benzene, 1,2,4-trichloro-5-((3-iodo-2-propynyl)oxy)-
18. Haloprogin (200 Mg)
19. Haloproginum
20. M-1028
21. Nsc100071
22. Haloprogine [inn-french]
23. Haloproginum [inn-latin]
24. Ncgc00181134-01
25. Haloprogina [inn-spanish]
26. 1,2,4-trichloro-5-((3-iodoprop-2-yn-1-yl)oxy)benzene
27. 1,2,4-trichloro-5-[(3-iodoprop-2-yn-1-yl)oxy]benzene
28. Benzene, 1,2,4-trichloro-5-[(3-iodo-2-propynyl)oxy]-
29. Halotex (tn)
30. Component Of Halotex
31. Einecs 212-286-6
32. 2,4,5-trichlorophenyl Gamma-iodopropargil Ether
33. 2,4,5-trichlorophenyl-gamma-iodopropargyl Ether
34. Unii-aiu7053owl
35. Nsc 100071
36. (3-iod-2-propinyl)-(2,4,5-trichlorphenyl)ether
37. Brn 1976771
38. Haloprogen
39. Haloprogin (jan/usan/inn)
40. Haloprogin [usan:usp:inn:jan]
41. Prestwick_232
42. Halotex (salt/mix)
43. Spectrum_001852
44. Haloprogin [mi]
45. Haloprogin [inn]
46. Haloprogin [jan]
47. Haloprogin [usan]
48. Haloprogin [vandf]
49. Ether,4,5-trichlorophenyl
50. Haloprogin [mart.]
51. Schembl3649
52. Haloprogin(200mg)
53. Dsstox_cid_26865
54. Dsstox_rid_81972
55. Haloprogin [who-dd]
56. Dsstox_gsid_46865
57. Kbioss_002369
58. 2,4,5-trichlorophenyl .gamma.-iodopropargyl Ether
59. Divk1c_000762
60. 1,2,4-trichloro-5-[(3-iodo-2-propynyl) Oxy]benzene
61. Chebi:5614
62. Benzene,1,2,4-trichloro-5-[(3-iodo-2-propynyl)oxy]
63. Dtxsid9046865
64. Haloprogin [orange Book]
65. Hms502g04
66. Kbio1_000762
67. Kbio2_002365
68. Kbio2_004933
69. Kbio2_007501
70. Wln: I1uu2or Bg Dg Eg
71. Ninds_000762
72. Hms3713a06
73. Bcp20218
74. Zinc1530649
75. Tox21_112741
76. Bdbm50194601
77. Ccg-220402
78. Db00793
79. 2,5-trichlorophenyl Iodopropargyl Ether
80. Idi1_000762
81. Ncgc00181134-02
82. Ncgc00181134-04
83. Cas-777-11-7
84. Hy-16246
85. Cs-0006262
86. 3-iodo-2-propynyl 2,5-trichlorophenyl Ether
87. D00339
88. 2,5-trichlorophenyl .gamma.-iodopropargil Ether
89. 2,5-trichlorophenyl .gamma.-iodopropargyl Ether
90. 777h117
91. 1,2,4-trichloro-5-(3-iodoprop-2-ynyloxy)benzene
92. Q5643439
93. 1,2,4-trichloro-5-(3-iodo-prop-2-ynyloxy)-benzene
94. Benzene,2,4-trichloro-5-[(3-iodo-2-propynyl)oxy]-
95. Brd-k13238168-001-01-2
| Molecular Weight | 361.4 g/mol |
|---|---|
| Molecular Formula | C9H4Cl3IO |
| XLogP3 | 4.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 2 |
| Exact Mass | 359.83725 g/mol |
| Monoisotopic Mass | 359.83725 g/mol |
| Topological Polar Surface Area | 9.2 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 247 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to treat fungal (Tinea) skin infections such as athlete's foot, jock itch, ringworm, and tinea versicolor.
Used as a topical ointment or cream in the treatment of Tinea infections. Tinea infections are superficial fungal infections caused by three species of fungi collectively known as dermatophytes (Trichophyton, Microsporum and Epidermophyton). Commonly these infections are named for the body part affected, including tinea corporis (general skin), tinea cruris (groin), and tinea pedis (feet).
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE11 - Haloprogin
Haloprogin is a halogenated phenolic ether administered topically for dermotaphytic infections. The mechanism of action is unknown, but is thought to be via inhibition of oxygen uptake and disruption of yeast membrane structure and function. There is a higher incidence of cutaneous side effects with haloprogin, including irritation, burning, vesiculation (blisters), scaling, and itching. It is generally used when the infection is unresponsive to other antifungals.
ABOUT THIS PAGE
24
PharmaCompass offers a list of Haloprogin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Haloprogin manufacturer or Haloprogin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Haloprogin manufacturer or Haloprogin supplier.
PharmaCompass also assists you with knowing the Haloprogin API Price utilized in the formulation of products. Haloprogin API Price is not always fixed or binding as the Haloprogin Price is obtained through a variety of data sources. The Haloprogin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Haloprogin manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Haloprogin, including repackagers and relabelers. The FDA regulates Haloprogin manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Haloprogin API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Haloprogin supplier is an individual or a company that provides Haloprogin active pharmaceutical ingredient (API) or Haloprogin finished formulations upon request. The Haloprogin suppliers may include Haloprogin API manufacturers, exporters, distributors and traders.
Haloprogin Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Haloprogin GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Haloprogin GMP manufacturer or Haloprogin GMP API supplier for your needs.
A Haloprogin CoA (Certificate of Analysis) is a formal document that attests to Haloprogin's compliance with Haloprogin specifications and serves as a tool for batch-level quality control.
Haloprogin CoA mostly includes findings from lab analyses of a specific batch. For each Haloprogin CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Haloprogin may be tested according to a variety of international standards, such as European Pharmacopoeia (Haloprogin EP), Haloprogin JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Haloprogin USP).

