
Synopsis
Synopsis
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
Canada

0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
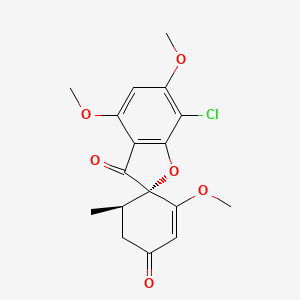

1. Fulvicin U F
2. Fulvicin-u-f
3. Fulvicinuf
4. Grifulvin V
5. Gris Peg
6. Gris-peg
7. Grisactin
8. Grisefuline
9. Grispeg
1. 126-07-8
2. (+)-griseofulvin
3. Amudane
4. Grisactin
5. Grisefuline
6. Griseofulvinum
7. Griseofulvina
8. Grizeofulvin
9. Grifulvin
10. Grisofulvin
11. Spirofulvin
12. Fulcin
13. Grysio
14. Lamoryl
15. Likuden
16. Fulvicin
17. Griseofulvine
18. Poncyl
19. Curling Factor
20. Grisovin
21. Grisactin V
22. Griseofulvine [inn-french]
23. Griseofulvinum [inn-latin]
24. Griseofulvina [inn-spanish]
25. Griseofulvin, Microcrystalline
26. Delmofulvina
27. Fulvistatin
28. Griscofulvin
29. Fulcine
30. Fulvina
31. Fulvinil
32. Fungivin
33. Greosin
34. Gresfeed
35. Grifulin
36. Grisetin
37. Guservin
38. Murfulvin
39. Neo-fulcin
40. Biogrisin-fp
41. Fulvican Grisactin
42. Griseofulvin-forte
43. Fulvicin-p/g
44. Fulvicin-u/f
45. Griseofulvin, Ultramicrocrystalline
46. Griseofulvin Microsize
47. Xuanjing
48. Mfcd00082343
49. Griseofulvin, Ultramicrosize
50. Usaf Sc-2
51. Griseomix
52. Grisactin Ultra
53. Nsc-755822
54. Griseofulvin Forte
55. Fulvicin P/g
56. 32hrv3e3d5
57. Chebi:27779
58. Griseofulvin Permeability Diameter
59. Nsc-34533
60. (2s,5'r)-7-chloro-3',4,6-trimethoxy-5'-methylspiro[1-benzofuran-2,4'-cyclohex-2-ene]-1',3-dione
61. Ncgc00091120-01
62. Dsstox_cid_674
63. Nsc 34533
64. Dsstox_rid_75725
65. Dsstox_gsid_20674
66. (1's,6'r)-7-chloro-2',4,6-trimethoxy-6'-methylspiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione
67. (1's-trans)- 7-chloro-2',4,6-trimethoxy-6'-methylspiro(benzofuran-2(3h),1'-cyclohex-2'-ene)-3,4'-dione
68. (2s,6'r)-7-chloro-2',4,6-trimethoxy-6'-methyl-3h,4'h-spiro[1-benzofuran-2,1'-cyclohex[2]ene]-3,4'-dione
69. (2s,6'r)-7-chloro-2',4,6-trimethoxy-6'-methyl-3h-spiro[benzofuran-2,1'-cyclohexan]-2'-ene-3,4'-dione
70. Ultragris-165
71. Ultragris-330
72. Fulvicin Bolus
73. Fulvicin-u/f (veterinary)
74. Fulvicin Bolus (veterinary)
75. Fulvicin P/g 165
76. Fulvicin P/g 330
77. Spiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione, 7-chloro-2',4,6-trimethoxy-6'-methyl-, (1's-trans)-
78. 7-chloro-4,6,2'-trimethoxy-6'-methylgris-2'-en-3,4'-dione
79. Caswell No. 471b
80. Fulvidex
81. (2s,6'r)-7-chloro-2',4,6-trimethoxy-6'-methyl-3h-spiro[1-benzofuran-2,1'-cyclohexan]-2'-ene-3,4'-dione
82. (1's,6'r)-7-chloro-2',4,6-trimethoxy-6'-methyl-3h-spiro[benzofuran-2,1'-cyclohex[2]ene]-3,4'-dione
83. Smr000718755
84. Ccris 320
85. Hsdb 1722
86. Sr-05000001535
87. Einecs 204-767-4
88. Epa Pesticide Chemical Code 471400
89. Griseofulvin And Alpha-ifn
90. Brn 0095226
91. Unii-32hrv3e3d5
92. Griseoflulvin
93. Griseofulvin, Microsize
94. Ai3-51015
95. Fulvicin Uf
96. Griseofulvin,(s)
97. Griseofulvin [usp:inn:ban:jan]
98. (2s,5'r)-7-chloro-3',4,6-trimethoxy-5'-methyl-spiro[benzofuran-2,4'-cyclohex-2-ene]-1',3-dione
99. Prestwick_247
100. Grisactin V (tn)
101. Gris-peg (tn)
102. Griseofulvin,microsize
103. Spectrum_000816
104. Cpd000718755
105. Specplus_000336
106. Prestwick3_000226
107. Spectrum2_000213
108. Spectrum3_000161
109. Spectrum4_000927
110. Spectrum5_000648
111. Griseofulvin [mi]
112. Griseofulvin [usp:inn]
113. Griseofulvin [inn]
114. Griseofulvin [jan]
115. Chembl562
116. Griseofulvin [hsdb]
117. Griseofulvin [iarc]
118. 7-chloro-2',4,6-trimethoxy-6'beta-methylspiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione
119. 7-chloro-4,6-dimethoxycoumaran-3-one-2-spiro-1'-(2'-methoxy-6'-methylcyclohex-2'-en-4'-one)
120. Griseofulvin [vandf]
121. Schembl21988
122. Bspbio_000271
123. Bspbio_001621
124. Griseofulvin [mart.]
125. Kbiogr_001454
126. Kbioss_001296
127. Spectrum200046
128. (2s-trans)-7-chloro-2',4,6-trimethoxy-6'-methylspiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione
129. 5-18-05-00150 (beilstein Handbook Reference)
130. Mls001304062
131. Mls002152905
132. Mls002154239
133. Spiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione, 7-chloro-2',4,6-trimethoxy-6'-methyl-, (2s-trans)-
134. Bidd:gt0024
135. Divk1c_000154
136. Divk1c_006432
137. Griseofulvin [usp-rs]
138. Griseofulvin [who-dd]
139. Griseofulvin [who-ip]
140. Griseofulvin (jan/usp/inn)
141. Spbio_000225
142. Bpbio1_000299
143. Megxm0_000184
144. Dtxsid8020674
145. Acon0_000953
146. Acon1_001843
147. Bdbm31775
148. Fulvicin-u/f Powder And Tablets
149. Hms500h16
150. Kbio1_000154
151. Kbio1_001376
152. Kbio2_001296
153. Kbio2_003864
154. Kbio2_006432
155. Kbio3_001121
156. Griseofulvin [green Book]
157. Ninds_000154
158. Griseofulvin [orange Book]
159. Hms1923e09
160. Hms2091a03
161. Hms2095n13
162. Hms2235f13
163. Hms3259d05
164. Hms3712n13
165. Interacts With Polymerized Microtubules And Associated Proteins
166. Pharmakon1600-00200046
167. Zinc622123
168. Griseofulvin [ep Monograph]
169. 7-chloro-2',4,6-trimethoxy-6'.beta.-methylspiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione
170. Spiro(benzofuran-2(3h),1'-(2)cyclohexene)-3,4'-dione, 7-chloro-2',4,6-trimethoxy-6'-methyl-, (1's,6'r)-
171. Griseofulvin [usp Monograph]
172. Tox21_111087
173. Tox21_202235
174. Tox21_303005
175. Ccg-38416
176. Lmpk13060001
177. Nsc755822
178. S4071
179. Griseofulvinum [who-ip Latin]
180. Akos015896380
181. Tox21_111087_1
182. Cs-3426
183. Db00400
184. Nc00616
185. Sdccgmls-0066450.p001
186. Griseofulvin 100 Microg/ml In Methanol
187. Idi1_000154
188. Ncgc00091120-02
189. Ncgc00091120-03
190. Ncgc00091120-04
191. Ncgc00091120-05
192. Ncgc00091120-06
193. Ncgc00091120-07
194. Ncgc00091120-08
195. Ncgc00091120-09
196. Ncgc00091120-13
197. Ncgc00256353-01
198. Ncgc00259784-01
199. As-13736
200. Bt164513
201. Hy-17583
202. Griseofulvin 100 Microg/ml In Acetonitrile
203. G0384
204. En300-52615
205. Bim-0051396.0001
206. C06686
207. D00209
208. Ab00052005_07
209. Griseofulvin, Vetranal(tm), Analytical Standard
210. Griseofulvin Permeability Diameter [usp-rs]
211. Q416096
212. Sr-01000837512
213. Griseofulvin, Antibiotic For Culture Media Use Only
214. Q-201178
215. Sr-01000837512-2
216. Sr-05000001535-1
217. Sr-05000001535-3
218. Sr-05000001535-4
219. Brd-k08273968-001-05-9
220. Brd-k08273968-001-09-1
221. Brd-k08273968-001-19-0
222. Z1258578352
223. Griseofulvin, British Pharmacopoeia (bp) Reference Standard
224. Griseofulvin, From Penicillium Griseofulvum, 97.0-102.0%
225. Griseofulvin, European Pharmacopoeia (ep) Reference Standard
226. Griseofulvin, United States Pharmacopeia (usp) Reference Standard
227. Griseofulvin Permeability Diameter, United States Pharmacopeia (usp) Reference Standard
228. Griseofulvin, Pharmaceutical Secondary Standard; Certified Reference Material
229. 107912-37-8
230. 7-chloro-2',4,6-trimethoxy-6'-methylspiro[benzofuran-2(3h),1',- [2]cyclohexene]-3,4'-dione
| Molecular Weight | 352.8 g/mol |
|---|---|
| Molecular Formula | C17H17ClO6 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 352.0713660 g/mol |
| Monoisotopic Mass | 352.0713660 g/mol |
| Topological Polar Surface Area | 71.1 Ų |
| Heavy Atom Count | 24 |
| Formal Charge | 0 |
| Complexity | 575 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 8 | |
|---|---|
| Drug Name | Grifulvin v |
| Active Ingredient | Griseofulvin, microsize |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 500mg; 125mg/5ml |
| Market Status | Prescription |
| Company | Valeant Luxembourg |
| 2 of 8 | |
|---|---|
| Drug Name | Griseofulvin |
| PubMed Health | Griseofulvin (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Griseofulvin microsize contains griseofulvin microsize for oral administration. The active ingredient, griseofulvin, is a fungistatic antibiotic, derived from a species of Penicillium. The chemical name of griseofulvin is 7-chloro-2', 4,6-trimethoxy- |
| Active Ingredient | Griseofulvin, microsize |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 250mg; 500mg; 125mg/5ml |
| Market Status | Prescription |
| Company | Vintage Pharms; Perrigo Co Tennessee; Sandoz; Ivax Sub Teva Pharms; Actavis Mid Atlantic; Sigmapharm Labs |
| 3 of 8 | |
|---|---|
| Drug Name | Griseofulvin, ultramicrosize |
| Active Ingredient | Griseofulvin, ultramicrosize |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Riconpharma |
| 4 of 8 | |
|---|---|
| Drug Name | Gris-peg |
| Drug Label | Gris-PEG Tablets contain ultramicrosize crystals of griseofulvin, an antibiotic derived from a species of Penicillium.Each Gris-PEG tablet contains:Active Ingredient: griseofulvin ultramicrosize .... 125 mgInactive Ingredients: colloidal silicon... |
| Active Ingredient | Griseofulvin, ultramicrosize |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Pedinol |
| 5 of 8 | |
|---|---|
| Drug Name | Grifulvin v |
| Active Ingredient | Griseofulvin, microsize |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 500mg; 125mg/5ml |
| Market Status | Prescription |
| Company | Valeant Luxembourg |
| 6 of 8 | |
|---|---|
| Drug Name | Griseofulvin |
| PubMed Health | Griseofulvin (By mouth) |
| Drug Classes | Antifungal |
| Drug Label | Griseofulvin microsize contains griseofulvin microsize for oral administration. The active ingredient, griseofulvin, is a fungistatic antibiotic, derived from a species of Penicillium. The chemical name of griseofulvin is 7-chloro-2', 4,6-trimethoxy- |
| Active Ingredient | Griseofulvin, microsize |
| Dosage Form | Tablet; Suspension |
| Route | Oral |
| Strength | 250mg; 500mg; 125mg/5ml |
| Market Status | Prescription |
| Company | Vintage Pharms; Perrigo Co Tennessee; Sandoz; Ivax Sub Teva Pharms; Actavis Mid Atlantic; Sigmapharm Labs |
| 7 of 8 | |
|---|---|
| Drug Name | Griseofulvin, ultramicrosize |
| Active Ingredient | Griseofulvin, ultramicrosize |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Riconpharma |
| 8 of 8 | |
|---|---|
| Drug Name | Gris-peg |
| Drug Label | Gris-PEG Tablets contain ultramicrosize crystals of griseofulvin, an antibiotic derived from a species of Penicillium.Each Gris-PEG tablet contains:Active Ingredient: griseofulvin ultramicrosize .... 125 mgInactive Ingredients: colloidal silicon... |
| Active Ingredient | Griseofulvin, ultramicrosize |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Pedinol |
Antibiotics, Antifungal
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
GRISEOFULVIN IS USED IN THE TREATMENT OF TINEAS (RINGWORM INFECTIONS) OF THE SKIN, HAIR & NAILS--TINEA CORPORIS, TINEA PEDIS, TINEA CRURIS, TINEA BARBAE, TINEA CAPITIS & TINEA UNGUIUM (ONYCHOMYCOSIS) CAUSED BY SUSCEPTIBLE SPECIES OF TRICHOPHYTON, MICROSPORUM, OR EPIDERMOPHYTON.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 83
MEDICATION (VET): DRUG IS USEFUL IN TREATMENT OF SUPERFICIAL FUNGUS INFECTIONS CAUSED BY TRICHOPHYTON MENTAGROPHYTES, RUBRUM, INTERDIGITALE, SCHOENLEINI, SULPHUREUM AND VERRUCOSUM, AND MICROSPORUM AND OVINI, CANIS, AND GYPSEUM...
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1166
MEDICATION (VET): TO TREAT RINGWORM OF SKIN OR NAILS ESPECIALLY THOSE AREAS DIFFICULT TO TREAT LOCALLY (EYE, MOUTH, NAIL AREAS), OR THOSE REFRACTORY TO OTHER THERAPY.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 247
For more Therapeutic Uses (Complete) data for GRISEOFULVIN (14 total), please visit the HSDB record page.
ORAL THRUSH DUE TO CANDIDAL OVERGROWTH HAS...OCCURRED.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 84
GRISEOFULVIN...IS CONTRAINDICATED IN PATIENTS WITH ACUTE INTERMITTENT PORPHYRIA OR A HISTORY OF.../IT/, HEPATOCELLULAR FAILURE, AND HYPERSENSITIVITY TO THE DRUG. ... SAFE USE...DURING PREGNANCY HAS NOT BEEN ESTABLISHED.
American Medical Association, Council on Drugs. AMA Drug Evaluations Annual 1994. Chicago, IL: American Medical Association, 1994., p. 1593
RARELY, TRANSIENT DIMINUTION OF HEARING HAS OCCURRED...PARESTHESIAS OF HANDS AND FEET HAS FOLLOWED EXTENDED THERAPY... OCCASIONALLY, LARGE DOSES HAVE PRODUCED...PSYCHOTIC SYMPTOMS.
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 84
GRISEOFULVIN HAS BEEN REPORTED TO CAUSE TACHYCARDIA AND FLUSHING...
McEvoy, G.K. (ed.). American Hospital Formulary Service--Drug Information 94. Bethesda, MD: American Society of Hospital Pharmacists, Inc. 1994 (Plus Supplements)., p. 84
For more Drug Warnings (Complete) data for GRISEOFULVIN (17 total), please visit the HSDB record page.
For the treatment of ringworm infections of the skin, hair, and nails, namely: tinea corporis, tinea pedis, tinea cruris, tinea barbae, cradle cap or other conditions caused by Trichophyton or Microsporum fungi.
FDA Label
Griseofulvin is a mycotoxic metabolic product of Penicillium spp. It was the first available oral agent for the treatment of dermatophytoses and has now been used for more than forty years. Griseofulvin is fungistatic with in vitro activity against various species of Microsporum Epidermophyton, and Trichophyton. It has no effect on bacteria or on other genera of fungi. Following oral administration, griseofulvin is deposited in the keratin precursor cells and has a greater affinity for diseased tissue. The drug is tightly bound to the new keratin which becomes highly resistant to fungal invasions. Once the keratin-Griseofulvin complex reaches the skin site of action, it binds to fungal microtubules (tubulin) thus altering fungal mitosis.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AA - Antibiotics
D01AA08 - Griseofulvin
D - Dermatologicals
D01 - Antifungals for dermatological use
D01B - Antifungals for systemic use
D01BA - Antifungals for systemic use
D01BA01 - Griseofulvin
Absorption
Poorly absorbed from GI ranging from 25 to 70% of an oral dose. Absorption is significantly enhanced by administration with or after a fatty meal.
IN RATS GIVEN ORAL DOSES OF 100 MG/KG BODY WT (36)CL-GRISEOFULVIN, 10% OF ACTIVITY WAS FOUND IN URINE AFTER 24 HR & 4% DURING 24-48 HR. ...IN ANOTHER STUDY, WITHIN 24-HR PERIOD ONLY 0.14% OF SIMILAR ORAL DOSES IN RATS WAS FOUND IN URINE, & 16% WAS RECOVERED IN FECES. FOLLOWING ITS IV INJECTION GRISEOFULVIN WAS DISTRIBUTED EVENLY THROUGHOUT TISSUES, ALTHOUGH HIGHER LEVELS WERE FOUND IN SKIN & LUNG.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 157 (1976)
Microsize - variable, ranging from 25 to 70% of an oral dose. Ultramicrosize - Almost completely absorbed. Absorption is significantly enhanced by administration with or after a fatty meal.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1457
Griseofulvin is deposited in varying concentrations in the keratin layer of the skin, hair, and nails. It can be detected in the stratum corneum of the skin within a few hours following administration. Only a very small fraction of an oral dose is distributed in the body fluids and tissues.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1457
Elimination: Renal. less than 1% of a dose is excreted as unchanged drug in the urine. Approximately 36% of griseofulvin is excreted unchanged in the feces.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1457
GRISEOFULVIN IS PROBABLY DEPOSITED IN BASAL CELLS AND IS CARRIED OUTWARDS INTO EPIDERMIS AS NORMAL SKIN GROWTH PROCEEDS. THIS...MAKES FOR LONG LATENCY FROM TIME MEDICATION IS BEGUN UNTIL EVIDENCE OF IMPROVEMENT OCCURS.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1166
Primarily hepatic with major metabolites being 6-methyl-griseofulvin and its glucuronide conjugate.
Griseofulvin is mainly metabolized to 6-dimethylgriseofulvin and its glucuronide.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 776
...IT HAS BEEN REPORTED THAT 6-DEMETHYLGRISEOFULVIN IS THE MAJOR URINARY METABOLITE...IN MAN, THE PREVIOUSLY REPORTED 4-DEMETHYL-GRISEOFULVIN WAS ABSENT. GRISEOFULVIC ACID (7-CHLORO-4,6-DIMETHOXY-6'-METHYLGRISAN-2',3,4'-TRIONE) WAS IDENTIFIED...
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 275
9-21 hours
DRUG HAS A HALF-LIFE IN PLASMA OF ABOUT 1 DAY, & APPROX 50% OF ORAL DOSE CAN BE DETECTED IN THE URINE WITHIN 5 DAYS, MOSTLY IN THE FORM OF METABOLITES /SRP: 36% IN THE FECES WITHIN 5 DAYS/.
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1173
The half-life of griseofulvin in canine plasma was found to be 47 minutes ... .
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 775
Griseofulvin is fungistatic, however the exact mechanism by which it inhibits the growth of dermatophytes is not clear. It is thought to inhibit fungal cell mitosis and nuclear acid synthesis. It also binds to and interferes with the function of spindle and cytoplasmic microtubules by binding to alpha and beta tubulin. It binds to keratin in human cells, then once it reaches the fungal site of action, it binds to fungal microtubes thus altering the fungal process of mitosis.
Fungistatic; griseofulvin inhibits fungal cell mitosis by causing disruption of the mitotic spindle structure, thereby arresting the metaphase of cell division. It is deposited in varying concentrations in the keratin precursor cells of skin, hair, and nails, rendering the keratin resistant to fungal invasion. As the infected keratin is shed, it is replaced with healthy tissue.
USP Convention. USPDI-Drug Information for the Health Care Professional. 14th ed. Volume I. Rockville, MD: United States Pharmacopeial Convention, Inc., 1994. (Plus Updates)., p. 1457

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
10
PharmaCompass offers a list of Griseofulvin API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Griseofulvin manufacturer or Griseofulvin supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Griseofulvin manufacturer or Griseofulvin supplier.
PharmaCompass also assists you with knowing the Griseofulvin API Price utilized in the formulation of products. Griseofulvin API Price is not always fixed or binding as the Griseofulvin Price is obtained through a variety of data sources. The Griseofulvin Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of GRISEOFULVIN, ULTRAMICROCRYSTALLINE, including repackagers and relabelers. The FDA regulates GRISEOFULVIN, ULTRAMICROCRYSTALLINE manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. GRISEOFULVIN, ULTRAMICROCRYSTALLINE API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE supplier is an individual or a company that provides GRISEOFULVIN, ULTRAMICROCRYSTALLINE active pharmaceutical ingredient (API) or GRISEOFULVIN, ULTRAMICROCRYSTALLINE finished formulations upon request. The GRISEOFULVIN, ULTRAMICROCRYSTALLINE suppliers may include GRISEOFULVIN, ULTRAMICROCRYSTALLINE API manufacturers, exporters, distributors and traders.
click here to find a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE DMF (Drug Master File) is a document detailing the whole manufacturing process of GRISEOFULVIN, ULTRAMICROCRYSTALLINE active pharmaceutical ingredient (API) in detail. Different forms of GRISEOFULVIN, ULTRAMICROCRYSTALLINE DMFs exist exist since differing nations have different regulations, such as GRISEOFULVIN, ULTRAMICROCRYSTALLINE USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE DMF submitted to regulatory agencies in the US is known as a USDMF. GRISEOFULVIN, ULTRAMICROCRYSTALLINE USDMF includes data on GRISEOFULVIN, ULTRAMICROCRYSTALLINE's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The GRISEOFULVIN, ULTRAMICROCRYSTALLINE USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE suppliers with USDMF on PharmaCompass.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP of the European Pharmacopoeia monograph is often referred to as a GRISEOFULVIN, ULTRAMICROCRYSTALLINE Certificate of Suitability (COS). The purpose of a GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of GRISEOFULVIN, ULTRAMICROCRYSTALLINE EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of GRISEOFULVIN, ULTRAMICROCRYSTALLINE to their clients by showing that a GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP has been issued for it. The manufacturer submits a GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP (COS) as part of the market authorization procedure, and it takes on the role of a GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP holder for the record. Additionally, the data presented in the GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the GRISEOFULVIN, ULTRAMICROCRYSTALLINE DMF.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. GRISEOFULVIN, ULTRAMICROCRYSTALLINE CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE suppliers with CEP (COS) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing GRISEOFULVIN, ULTRAMICROCRYSTALLINE as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for GRISEOFULVIN, ULTRAMICROCRYSTALLINE API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture GRISEOFULVIN, ULTRAMICROCRYSTALLINE as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain GRISEOFULVIN, ULTRAMICROCRYSTALLINE and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a GRISEOFULVIN, ULTRAMICROCRYSTALLINE NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE suppliers with NDC on PharmaCompass.
GRISEOFULVIN, ULTRAMICROCRYSTALLINE Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of GRISEOFULVIN, ULTRAMICROCRYSTALLINE GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right GRISEOFULVIN, ULTRAMICROCRYSTALLINE GMP manufacturer or GRISEOFULVIN, ULTRAMICROCRYSTALLINE GMP API supplier for your needs.
A GRISEOFULVIN, ULTRAMICROCRYSTALLINE CoA (Certificate of Analysis) is a formal document that attests to GRISEOFULVIN, ULTRAMICROCRYSTALLINE's compliance with GRISEOFULVIN, ULTRAMICROCRYSTALLINE specifications and serves as a tool for batch-level quality control.
GRISEOFULVIN, ULTRAMICROCRYSTALLINE CoA mostly includes findings from lab analyses of a specific batch. For each GRISEOFULVIN, ULTRAMICROCRYSTALLINE CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
GRISEOFULVIN, ULTRAMICROCRYSTALLINE may be tested according to a variety of international standards, such as European Pharmacopoeia (GRISEOFULVIN, ULTRAMICROCRYSTALLINE EP), GRISEOFULVIN, ULTRAMICROCRYSTALLINE JP (Japanese Pharmacopeia) and the US Pharmacopoeia (GRISEOFULVIN, ULTRAMICROCRYSTALLINE USP).
