



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0

1. Cystorelin
2. Dirigestran
3. Factrel
4. Fsh Releasing Hormone
5. Fsh-releasing Hormone
6. Gn-rh
7. Gnrh
8. Gonadoliberin
9. Gonadorelin
10. Gonadorelin Acetate
11. Gonadorelin Hydrochloride
12. Gonadotropin Releasing Hormone
13. Gonadotropin-releasing Hormone
14. Kryptocur
15. Lfrh
16. Lh Fsh Releasing Hormone
17. Lh Releasing Hormone
18. Lh-fsh Releasing Hormone
19. Lh-releasing Hormone
20. Lh-rh
21. Lhfsh Releasing Hormone
22. Lhfshrh
23. Lhrh
24. Luliberin
25. Luteinizing Hormone Releasing Hormone
26. Luteinizing Hormone-releasing Hormone
27. Releasing Hormone, Lhfsh
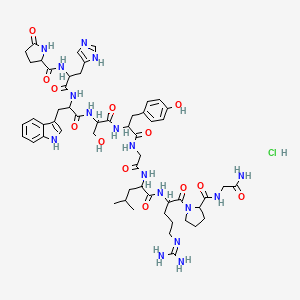
1. Gonadorelin Hydrochloride
| Molecular Weight | 1218.7 g/mol |
|---|---|
| Molecular Formula | C55H76ClN17O13 |
| Hydrogen Bond Donor Count | 17 |
| Hydrogen Bond Acceptor Count | 15 |
| Rotatable Bond Count | 31 |
| Exact Mass | 1217.5497032 g/mol |
| Monoisotopic Mass | 1217.5497032 g/mol |
| Topological Polar Surface Area | 475 Ų |
| Heavy Atom Count | 86 |
| Formal Charge | 0 |
| Complexity | 2390 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 8 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


