
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz

1. Doriden
2. Noxiron
1. Doriden
2. Elrodorm
3. Noxyron
4. Sarodormin
5. Glutethimid
6. Glutetimid
7. Noxiron
8. Rigenox
9. Glutathimid
10. Glutetimide
11. Gluthetimide
12. Glimid
13. Alfimid
14. Ondasil
15. Gimid
16. 2-ethyl-2-phenylglutarimide
17. 77-21-4
18. Doriden-sed
19. Glutethimidum
20. Glutetimidu
21. 2,6-piperidinedione, 3-ethyl-3-phenyl-
22. Glutarimide, 2-ethyl-2-phenyl-
23. 3-ethyl-3-phenyl-2,6-piperidinedione
24. 3-ethyl-3-phenylpiperidine-2,6-dione
25. 2-phenyl-2-ethylglutaric Acid Imide
26. 3-ethyl-3-phenyl-2,6-dioxopiperidine
27. 3-phenyl-3-ethyl-2,6-dioxopiperidine
28. 3-ethyl-3-phenyl-2,6-diketopiperidine
29. 3-phenyl-3-ethyl-2,6-diketopiperidine
30. Cc 11511
31. Phenyl-aethyl-glutarsaeureimid
32. Nsc 95239
33. Nsc-95239
34. C8i4bvn78e
35. Chebi:5439
36. .alpha.-ethyl-.alpha.-phenylglutarimide
37. .alpha.-phenyl-.alpha.-ethylglutarimide
38. Ncgc00159407-02
39. Ncgc00159407-04
40. Glutetimida
41. Dorimide
42. Glutetimide [dcit]
43. Glutetimidu [polish]
44. Dl-glutethimide
45. Dsstox_cid_3102
46. .alpha.-phenyl-.alpha.-ethylglutaric Acid Imide
47. Dsstox_rid_76876
48. Dsstox_gsid_23102
49. Glutethimidum [inn-latin]
50. Glutetimida [inn-spanish]
51. Alpha-ethyl-alpha-phenylglutarimide
52. Alpha-phenyl-alpha-ethylglutarimide
53. Cas-77-21-4
54. Doriden (tn)
55. Phenyl-aethyl-glutarsaeureimid [german]
56. Hsdb 3088
57. Alpha-phenyl-alpha-ethylglutaric Acid Imide
58. Einecs 201-012-0
59. Glutethimide (jan/inn)
60. Unii-c8i4bvn78e
61. Glutethamide
62. Glutethimide [usp:inn:ban]
63. Ai3-50601
64. Dea No. 2550
65. Glutethimide [mi]
66. Glutethimide [inn]
67. Glutethimide [jan]
68. 2, 3-ethyl-3-phenyl-
69. Glutethimide [hsdb]
70. Chembl1102
71. Glutethimide [vandf]
72. Glutethimide [mart.]
73. Glutethimide [who-dd]
74. Schembl113708
75. Wln: T6vmvtj D2 Dr
76. Gtpl7192
77. Dtxsid1023102
78. Glutethimide [orange Book]
79. Nsc95239
80. Tox21_111641
81. Akos015897742
82. Tox21_111641_1
83. Db01437
84. Ncgc00159407-03
85. Ac-16077
86. C07489
87. D00532
88. 056g091
89. Q414236
90. 3-ethyl-6-hydroxy-3-phenyl-2,3,4,5-tetrahydropyridin-2-one
91. Glutethimide Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
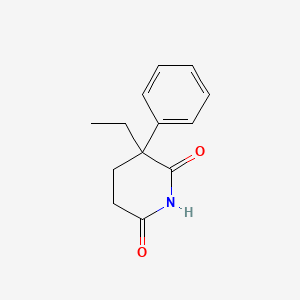
| Molecular Weight | 217.26 g/mol |
|---|---|
| Molecular Formula | C13H15NO2 |
| XLogP3 | 1.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 217.110278721 g/mol |
| Monoisotopic Mass | 217.110278721 g/mol |
| Topological Polar Surface Area | 46.2 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 294 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Sedatives, Nonbarbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
The primary Medication Classification of the US Veterans Administration is CN309: Sedatives/Hypnotics, Other.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Used for the short-term treatment of insomnia; however, it has been replaced by safer and more effective sedative-hyponotic agents. /Not Included in US product label/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
Adverse effects of glutethimide may include gastric irritation, nausea, hiccups, dry mouth, diarrhea, and blurring of vision. Rash including fixed eruptions, purpuric or urticarial rash, or, rarely, exfoliative dermatitis may also occur. If skin rash appears, glutethimide should be discontinued. Glutethimide induced skin rash ususally disappears within 2-3 days after withdrawal of the drug. Residual sedation or "hangover," paradoxical excitation, headache, and vertigo have also been reported with glutethimide. Rarely, acute hypersensitivity reactions, blood dyscrasias (including thrombocytopenic purpura, aplastic anemia, leukopenia, and megaloblastic anemia), peripheral neuropathy, acute intermittent porphyria, and jaundice have occurred. One case of osteomalacia has been attributed to long-term use of glutethimide; concentrations of various serum enzymes including alkaline phosphatase returned to normal when the drug was withdrawn.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
Rarely, acute hypersensitivity reactions, blood dyscrasias (including thrombocytopenic purpura, aplastic anemia, leukopenia, and megaloblastic anemia), peripheral neuropathy, acute intermittent porphyria, and jaundice have occurred. One case of osteomalacia has been attributed to glutethimide; concentrations of various serum enzymes including alkaline phosphatase returned to normal when the drug was withdrawn.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
Glutethmide should be used with caution in patients having conditions which might be aggravated by the drug's anticholinergic activity such as prostatic hypertrophy, stenosing peptic ulcer, pyloroduodenal obstruction, bladder neck obstruction, angle-closure glaucoma, or cardiac arrhythmias.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
Patients should be warned that glutethimide may impair ability to perform hazardous activities requiring mental alertness or physical coordination (e.g., operating machinery, driving a moter vehicle). Glutethimide should be used cautiously in patients who are mentally depressed, have sucidal tendencies or histories of drug abuse, or whose histories indicate that they may increase dosage on their own initiative. Large quantities of the drug should not be prescribed.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
For more Drug Warnings (Complete) data for GLUTETHIMIDE (13 total), please visit the HSDB record page.
A seriously toxic (potentially lethal) serum level of glutethimide is 3 mg/dL or greater, and reported fatal serum levels of glutethimide range between 2 and 88 mg/dL.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 842
For the treatment of insomnia.
Glutethimide, like the barbiturates, is a hypnotic sedative. It was introduced in 1954 as a safer alternative to barbiturates but was soon determined to be just as likely to cause addiction and withdrawal symptoms.
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CE - Piperidinedione derivatives
N05CE01 - Glutethimide
Absorption
Variable
Route of Elimination
glutethimide is inactivated by conjugation and the metabolites are excreted in urine, only 2% of the parent substance is excreted in urine, up to 2% of the dose has been reported to be found in the faeces.
Glutethimide appears to be irregularly absorbed from the GI tract. Plasma concentrations of the drug required for sedative or hypnotic effects are not known. In limited studies, oral administration of 500 mg and 1 g single doses of glutethimide produced peak plasma concentrations of 2.9-7.1 ug/mL and 6.2-6.8 ug/mL, respectively, within 1-6 hours. The onset of action of glutethimide is rapid; sleep is usually induced within 30 minutes and lasts 4-8 hours following usual hypnotic doses.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2102
Once absorbed, glutethimide quickly becomes concentrated in organs containing fat such as brain and adipose tissues, with a volume of distribution larger than that of whole body water. The drug recirculates from fat stores and is carried to the liver where it undergoes biotransformation, somewhat reminiscent of redistribution of ultra short acting barbiturates like thiopental.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 839
Distribution studies indicate that there is extensive tissue localization of glutethimide, particularly in adipose tissue. In addition, glutethimide (and/or its metabolites) has been detected in liver, kidneys, brain, and bile. Glutethimide crosses the placenta, and small quantities of the drug are distributed into milk. When 1 g doses of glutethimide were administered to pregnant women 2 hours before delivery, maternal and neonatal plasma concentrations of glutethimide were essentially the same immediately after delivery.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2102
In vitro, about 54% of the drug appears to be bound to plasma proteins.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2102
For more Absorption, Distribution and Excretion (Complete) data for GLUTETHIMIDE (12 total), please visit the HSDB record page.
Hepatic. Glutethimide is almost completely metabolized.
Hepatic biotransformation. Glutethimide is almost completely metabolized.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
The metabolic fate of glutehimide has not been completely elucidated, particularly following toxic doses of the drug. There is considerable evidence that therapeutic doses of glutethimide are almost completely metabolized in the liver by hydroxylation of the ethyl side chain (levorotatory isomer) and excreted in urine chiefly as glucuronides. About 2% of a dose is metabolized to glutaconimide which has some hypnotic activity. ... 4-Hydroxy-2-ethyl-2 phenylglutarimide is a metabolite which accumulates in the plasma and tissues (including the brain) of patients who have ingested large doses of glutethimide. Several phenolic metabolites and others yet to be identified have been found in urine following acute glutethimide overdosage.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
Although glutethimide blood concentrations greater than 10 ug/mL are generally associated with intoxication, there is a poor correlation between glutethimide plasma concentration and the clinical course of the patient, possibly because of the formation and accumulation of an active metabolite, 4-hyroxy-2-ethyl-2-phenylglutarimide.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
Glutethimide has an asymmetric carbon atom and gives two optical isomers, both of which are metabolized in a different fashion. The D-isomer is hydrolyzed at the glutarimide ring, loses water, and breaks down into alpha-phenyl-alpha-ethylglutaconimide, which is excreted in the urine as a nonconjugated metabolite in approximately 2% of the administered dose. A major portion of the hydrolyzed D-isomer is combined with glucuronic acid and is excreted in the urine in approximately 45% of the administered dose. The L-isomer is hydrolyzed with release of acetaldehyde from a-phenyl glutarimide. This metabolite is isolated in the urine in approximately 4 percent of the administered dose. The remaining major portion is also combined with glucuronic acid and is excreted in the urine in approximately 45 % of the administered dose. Both glucuronides are water soluble but not fat soluble and no longer possess sedative activity.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 840
For more Metabolism/Metabolites (Complete) data for GLUTETHIMIDE (7 total), please visit the HSDB record page.
10-12 hours
Half-life about 10 to 12 hours.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2007.
The decline of serum concentrations of glutethimide is biphasic; the half-life for the first phase is about 4 hrs and the half-life for the second phase is 10-12 hours. The apparent serum half-life of the drug in patients severely intoxicated with glutethimide has been reported to be prolonged, but this may have been due to continued absorption of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2103
In a series of patients with glutethimide overdose, mean serum half-life has been found to be prolonged to around 40.1 hr. The definition of biologic half-life for glutethimide is somewhat difficult because of the rapidly appearing inactive metabolites (glucuronides). The half-life of the inactivated dose (effective biologic half-life) and the time when half of the dose is excreted (maximal biologic half-life) are different; the effective biologic half-life is approximately 10 hr, and the maximal biologic half-life is approximately 16 hr.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 841
The spontaneous decline of glutethimide blood levels follows a biphasic curve, with an initial rapid phase and a later slow phase. During the initial rapid phase, a mean serum half-life of 3.9 hr is obtained... The decline in drug concentration during the second, slower phase gives a mean serum half-life of 11.6 hr, this being brought about by loss of drug from tissue compartments and blood (or central compartment) through metabolism and excretion.
Haddad, L.M., Clinical Management of Poisoning and Drug Overdose. 2nd ed. Philadelphia, PA: W.B. Saunders Co., 1990., p. 841
Glutethimide seems to be a GABA agonist which helps induce sedation. It also induces CYP 2D6. When taken with codeine, it enables the body to convert higher amounts of codeine (higher than the average 5 - 10%) to morphine. This combination of effects enhances sedation.
Glutethimide has CNS depressant effects similar to those of the barbiturates. The mechanism of action of the drug is not known. In doses used for hypnosis, glutethimide produces cerebral depression and quiet, deep sleep. Like barbiturates, usual hypnotic doses of glutethimide significantly suppress rapid eye movement (REM) or dreaming stage of sleep. Although some tolerance develops to the REM-suppressant effects during intermediate-term chronic administration, REM rebound occurs when the drug is withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2102
Glutethimide exhibits anticholinergic activity manifested by mydriasis and inhibition of salivary secretions and intestinal motility. Toxic doses of the drug often produce marked mydriasis, adynamic ileus, and urinary bladder atony. Hypnotic doses of glutethimide do not produce reliable analgesic, antipyretic, anticonvulsant, antiemetic, or antitussive effects. Glutethimide induces liver microsomal enzymes and thus may alter the metabolism of other drugs.
McEvoy, G.K. (ed.). American Hospital Formulary Service - Drug Information 1999. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1999 (Plus Supplements)., p. 2102
Glutethimide directly blocks electron transfer in cellular respiration.
WHO; Poisons Information Monographs (PIMs) for Glutethimide (PIM 246) (1998). Avalable from: https://www.inchem.org/pages/pims.html as of March 27, 2007.
ABOUT THIS PAGE
88
PharmaCompass offers a list of Glutethimide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Glutethimide manufacturer or Glutethimide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Glutethimide manufacturer or Glutethimide supplier.
PharmaCompass also assists you with knowing the Glutethimide API Price utilized in the formulation of products. Glutethimide API Price is not always fixed or binding as the Glutethimide Price is obtained through a variety of data sources. The Glutethimide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Glutethimide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Glutethimide, including repackagers and relabelers. The FDA regulates Glutethimide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Glutethimide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Glutethimide supplier is an individual or a company that provides Glutethimide active pharmaceutical ingredient (API) or Glutethimide finished formulations upon request. The Glutethimide suppliers may include Glutethimide API manufacturers, exporters, distributors and traders.
click here to find a list of Glutethimide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Glutethimide DMF (Drug Master File) is a document detailing the whole manufacturing process of Glutethimide active pharmaceutical ingredient (API) in detail. Different forms of Glutethimide DMFs exist exist since differing nations have different regulations, such as Glutethimide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Glutethimide DMF submitted to regulatory agencies in the US is known as a USDMF. Glutethimide USDMF includes data on Glutethimide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Glutethimide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Glutethimide suppliers with USDMF on PharmaCompass.
Glutethimide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Glutethimide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Glutethimide GMP manufacturer or Glutethimide GMP API supplier for your needs.
A Glutethimide CoA (Certificate of Analysis) is a formal document that attests to Glutethimide's compliance with Glutethimide specifications and serves as a tool for batch-level quality control.
Glutethimide CoA mostly includes findings from lab analyses of a specific batch. For each Glutethimide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Glutethimide may be tested according to a variety of international standards, such as European Pharmacopoeia (Glutethimide EP), Glutethimide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Glutethimide USP).

