



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
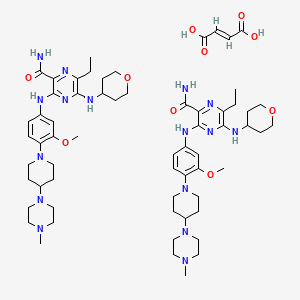

1. Gilteritinib Hemifumarate
2. Asp-2215 Hemifumarate
3. 1254053-84-3
4. Gilteritinib Fumarate [usan]
5. Asp2215 Hemifumarate
6. 5rzz0z1gjt
7. 1254053-84-3 (hemifumarate)
8. 2-pyrazinecarboxamide, 6-ethyl-3-((3-methoxy-4-(4-(4-methyl-1-piperazinyl)-1-piperidinyl)phenyl)amino)-5-((tetrahydro-2h-pyran-4-yl)amino)-, (2e)-2-butenedioate (2:1)
9. 2-pyrazinecarboxamide, 6-ethyl-3-[[3-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]amino]-5-[(tetrahydro-2h-pyran-4-yl)amino]-, (2e)-2-butenedioate (2:1)
10. 6-ethyl-3-((3-methoxy-4-(4-(4-methylpiperazin-1-yl)piperidin-1-yl)phenyl)amino)-5-((tetrahydro-2h-pyran-4-yl)amino)pyrazine-2-carboxamide Hemifumarate
11. Unii-5rzz0z1gjt
12. Xospata (tn)
13. (e)-but-2-enedioic Acid;6-ethyl-3-[3-methoxy-4-[4-(4-methylpiperazin-1-yl)piperidin-1-yl]anilino]-5-(oxan-4-ylamino)pyrazine-2-carboxamide
14. Chembl3301603
15. Schembl21819929
16. Dtxsid001027950
17. Gilteritinib Fumarate (jan/usan)
18. Gilteritinib Fumarate [jan]
19. Gilteritinib Fumarate [who-dd]
20. Gilteritinib Fumarate [orange Book]
21. D10800
22. Q27262795
| Molecular Weight | 1221.5 g/mol |
|---|---|
| Molecular Formula | C62H92N16O10 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 24 |
| Rotatable Bond Count | 20 |
| Exact Mass | 1220.71823320 g/mol |
| Monoisotopic Mass | 1220.71823320 g/mol |
| Topological Polar Surface Area | 317 Ų |
| Heavy Atom Count | 88 |
| Formal Charge | 0 |
| Complexity | 904 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Xospata is indicated as monotherapy for the treatment of adult patients who have relapsed or refractory acute myeloid leukaemia (AML) with a FLT3 mutation.
L01EX13


