



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
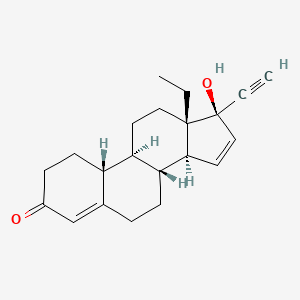

1. 13-ethyl-17-hydroxy-18,19-dinor-17 Alpha-pregna-4,15-dien-20-yn-3-one
2. 17-alpha-ethinyl-13-ethyl-17 Beta-hydroxy-4,15-gonadien-3-one
3. 18,19-dinorpregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-, (17-alpha)-
4. Gestoden
5. Gestodene, ((17alpha)-(+-))-isomer
6. Sh B 3331
1. 60282-87-3
2. Gestoden
3. Gestodenum [inn-latin]
4. Gestodeno [inn-spanish]
5. Sh B 331
6. Sh-b-331
7. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14-decahydrocyclopenta[a]phenanthren-3-one
8. 1664p6e6mi
9. Gestinol
10. 18,19-dinorpregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-, (17a)-
11. Dsstox_cid_26478
12. Dsstox_rid_81649
13. Dsstox_gsid_46478
14. Gestodenum
15. Gestodeno
16. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-6,7,8,9,10,11,12,13,14,17-decahydro-1h-cyclopenta[a]phenanthren-3(2h)-one
17. Gestodene [usan:inn:ban]
18. Cas-60282-87-3
19. Ccris 9189
20. Hsdb 3594
21. Einecs 262-145-8
22. Brn 4237181
23. Unii-1664p6e6mi
24. Gestodene- Bio-x
25. Ncgc00164611-01
26. Gestodene [inn]
27. Gestodene [mi]
28. Gestodene (usan/inn)
29. Gestodene [hsdb]
30. Gestodene [usan]
31. 17-alpha-ethinyl-13-ethyl-17-beta-hydroxy-4,15-gonadien-3-one
32. Gestodene [mart.]
33. 13-ethyl-17-hydroxy-18,19-dinor-17alpha-pregna-4,15-dien-20-yn-3-one
34. Gestodene [who-dd]
35. 18,19-dinorpregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-, (17-alpha)-
36. Schembl37271
37. Gestodene [ep Impurity]
38. Gestodene For System Suitability
39. Chembl1213583
40. Dtxsid6046478
41. Gestodene [ep Monograph]
42. Gestodene, >=98% (hplc)
43. Gtpl11246
44. Shb 331
45. Shb-331
46. Sht-546
47. Chebi:135323
48. Wl 70
49. Bcp22665
50. Hy-b0110
51. Tox21_112232
52. Ac-474
53. Bdbm50247973
54. Mfcd00867858
55. S1376
56. Sh-546
57. Akos024462671
58. Femodene (ethinylestradiol, Gestodene)
59. Tox21_112232_1
60. Zinc238809420
61. Ccg-267584
62. Cs-1862
63. Db06730
64. Millinette (ethinylestradiol, Gestodene)
65. Pregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-18,19-dinor-, (17alpha)-
66. Ncgc00263565-01
67. Ac-20038
68. As-13029
69. Bg164505
70. G0404
71. D04316
72. Ab01274782-01
73. Ab01274782_02
74. 282g873
75. A832668
76. Q408289
77. Q-101426
78. Brd-k97197005-001-03-2
79. Gestodene, European Pharmacopoeia (ep) Reference Standard
80. 17alpha -ethynyl-17beta-hydroxy-18-methyl-4,15-estradien-3-one
81. 17alpha-ethynyl-17beta-hydroxy-18-methyl-4,15-estradien-3-one
82. 17alpha-ethynyl-17beta-hydroxy-18-methyl-estra-4,15-dien-3-one
83. 13beta-ethyl-17beta-hydroxy-18,19-dinorpregna-4,15-dien-20-yn-3-one
84. 13-ethyl-17-hydroxy-18,19-dinor-17.alpha.-pregna-4,15-dien-20-yn-3-one
85. Pregna-4,15-dien-20-yn-3-one, 13-ethyl-17-hydroxy-18,19-dinor-, (17.alpha.)-
86. (1s,2r,10r,11s,14r,15s)-15-ethyl-14-ethynyl-14-hydroxytetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-6,12-dien-5-one
87. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,13,14,17-dodecahydro-3h-cyclopenta[a]phenanthren-3-one
88. (8r,9s,10r,13s,14s,17r)-13-ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,14-decahydrocyclopenta[a]phenanthren-3-one;gestodene
| Molecular Weight | 310.4 g/mol |
|---|---|
| Molecular Formula | C21H26O2 |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 310.193280068 g/mol |
| Monoisotopic Mass | 310.193280068 g/mol |
| Topological Polar Surface Area | 37.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 648 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 6 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Contraceptives, oral, Synthetic; Progestational hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
/Triadene is indicated for/ oral contraception and the recognized gynecological indications for such estrogen-progestogen combinations.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
Some epidemiological studies have suggested an association between the use of combined oral contraceptives (COCs) and an increased risk of arterial and venous thrombotic and thromboembolic diseases such as myocardial infarction, stroke, deep venous thrombosis and pulmonary embolism. These events occur rarely. Full recovery from such disorders does not always occur, and it should be realised that in a few cases they are fatal.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
/Contraindications for Triadene include/: Pregnancy; severe disturbances of liver function, jaundice or persistent itching during a previous pregnancy, Dubin-Johnson syndrome, Rotor syndrome, previous or existing liver tumors; history of confirmed venous thromboembolism (VTE). Family history of idiopathic VTE. Other known risk factors for VTE; existing or previous arterial thrombotic or embolic processes, conditions which predispose to them eg disorders of the clotting processes, valvular heart disease and atrial fibrillation; Sickle-cell anemia; mammary or endometrial carcinoma, or a history of these conditions; severe diabetes mellitus with vascular changes; disorders of lipid metabolism; history of herpes gestationis; deterioration of otosclerosis during pregnancy; undiagnosed abnormal vaginal bleeding; hypersensitivity to any of the components of Triadene.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
The following conditions require strict medical supervision during medication with oral contraceptives. Deterioration or first appearance of any of these conditions may indicate that use of the oral contraceptive should be discontinued: diabetes mellitus, or a tendency towards diabetes mellitus (eg unexplained glycosuria), hypertension, varicose veins, a history of phlebitis, otosclerosis, multiple sclerosis, epilepsy, porphyria, tetany, disturbed liver function, Sydenham's chorea, renal dysfunction, family history of clotting disorders, obesity, family history of breast cancer and patient history of benign breast disease, history of clinical depression, systemic lupus erythematosus, uterine fibroids and migraine, gall-stones, cardiovascular diseases, chloasma, asthma, an intolerance to contact lenses, or any disease that is prone to worsen during pregnancy.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
In rare cases, headaches, gastric upsets, nausea, vomiting, breast tenderness, changes in body weight, changes in libido, depressive moods can occur.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
For more Drug Warnings (Complete) data for GESTODENE (44 total), please visit the HSDB record page.
Contraceptives, Oral, Hormonal
Oral contraceptives which owe their effectiveness to hormonal preparations. (See all compounds classified as Contraceptives, Oral, Hormonal.)
Contraceptives, Oral, Synthetic
Oral contraceptives which owe their effectiveness to synthetic preparations. (See all compounds classified as Contraceptives, Oral, Synthetic.)
Progestins
Compounds that interact with PROGESTERONE RECEPTORS in target tissues to bring about the effects similar to those of PROGESTERONE. Primary actions of progestins, including natural and synthetic steroids, are on the UTERUS and the MAMMARY GLAND in preparation for and in maintenance of PREGNANCY. (See all compounds classified as Progestins.)
G03AA10
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
Absorption
in vitro 99% using 3H=R5020 / in vivo similar to progesterone
Orally administered gestodene is rapidly and completely absorbed.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
The absolute bioavailability of gestodene was determined to be 99% of the dose administered.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
For gestodene, an apparent volume of distribution of 0.7 L/kg and a metabolic clearance rate from serum of about 0.8 mL/min/kg were determined.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
Following ingestion of 0.1 mg gestodene together with 0.03 mg ethinylestradiol (which represents the combination with the highest gestodene content of the tri-step formulation), maximum drug serum levels of about 5.6 ng/mL are reached at 0.5 hour.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
For more Absorption, Distribution and Excretion (Complete) data for GESTODENE (8 total), please visit the HSDB record page.
The biotransformation follows the known pathways of steroid metabolism. No pharmacologically active metabolites are known.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
Gestodene is metabolized primarily in the liver by CYP 3A4 and is a strong inducer of this enzyme. Although ethinylestradiol is also metabolized by CYP 3A4, gestodene does not appear to inhibit its metabolism. Known metabolites of gestodene include dihydrogestodene, 3,5-tetrahydrogestodene and hydroxygestodene.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 149 (2007)
There is limited information on the metabolism of levonorgestrel, norethindrone and structurally related contraceptive steroids. Both levonorgestrel and norethindrone undergo extensive reduction of the alpha, beta-unsaturated ketone in ring A. Levonorgestrel also undergoes hydroxylation at carbons 2 and 16. The metabolites of both compounds circulate predominantly as sulfates. In urine, levonorgestrel metabolites are found primarily in the glucuronide form, whereas norethindrone metabolites are present in approximately equal amounts as sulfates and glucuronides. Of the progestogens structurally related to norethindrone, norethindrone acetate, ethynodiol diacetate, norethindrone enanthate, and perhaps lynestrenol, undergo rapid hydrolysis and are converted to the parent compound and its metabolites. There is no convincing evidence that norethynodrel is converted to norethindrone. Of the progestogens structurally related to levonorgestrel, it appears that neither desogestrel nor gestodene are transformed to the parent compound. However, there is evidence that norgestimate can be, at least partly, converted to levonorgestrel. ...
PMID:2143719 Stanczyk FZ, Roy S; Contraception 42 (1): 67-96 (1990)
16 to 18 hrs.
Gestodene serum levels decrease in two phases, characterised by half-lives of 0.1 hours and about 18 hours.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
The half-life of elimination of gestodene was shown to range from 12 to 14 hr for the three doses studied (0.025, 0.075 or 0.125 mg gestodene).
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 149 (2007)
Gestodene is not excreted in unchanged form but as metabolites, which are eliminated with a half-life of about 1 day.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
This estrogen-progestogen combination acts by inhibiting ovulation by suppression of the mid-cycle surge of luteinizing hormone, the inspissation of cervical mucus so as to constitute a barrier to sperm, and the rendering of the endometrium unreceptive to implantation.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Triadene (Last updated December 2007). Available from, as of March 25, 2011: https://www.medicines.org.uk/EMC/medicine/1847/SPC/Triadene/
Gestodene, one of three new gonane progestins, is the most potent on a per weight basis in regard to progestational effects and has little or no estrogenic effect. In in vivo animal studies, gestodene also has less androgenic activity compared with progestins found in older combination oral contraceptive formulations. It binds to mineralocorticoid receptors and consequently is a competitive aldosterone inhibitor, leading to speculation that it may be beneficial in hypertensive patients. ...In general, studies show that the incidence of side effects associated with the progestin and estrogen components tends to be low, with very little impact on lipid and carbohydrate metabolism. ...
PMID:8178907 Shoupe D; Am J Obstet Gynecol 170 (5 Pt 2): 1562-8 (1994)
Gestodene (17 alpha-ethynyl-13 beta-ethyl-17 beta-hydroxy-4, 15-gonadien-3-one) is the most potent synthetic progestin currently available and it is widely used as a fertility regulating agent in a number of contraceptive formulations because of its high effectiveness, safety and acceptability. The observation that contraceptive synthetic progestins exert hormone-like effects other than their progestational activities, prompted us to investigate whether gestodene (GSD) administration may induce estrogenic effects, even though the GSD molecule does not interact with intracellular estrogen receptors (ER). To assess whether GSD may exert estrogenic effects through some of its neutral metabolites, a series of experimental studies were undertaken using GSD and three of its A-ring reduced metabolites. Receptor binding studies by displacement analysis confirmed that indeed GSD does not bind to the ER, whereas its 3 beta,5 alpha-tetrahydro reduced derivative (3 beta GSD) interacts with a relative high affinity with the ER. The 3 alpha,5 alpha GSD isomer (3 alpha GSD) also binds to the ER, though to a lesser extent. The ability of the A-ring reduced GSD derivatives to induce estrogenic actions was evaluated by the use of two different molecular bioassays: (a) transactivation of a yeast system co-transfected with the human ER alpha (hER alpha) gene and estrogen responsive elements fused to the beta-galactosidase reporter vector and (b) transactivation of the hER alpha-mediated transcription of the chloramphenicol acetyl transferase (CAT) reporter gene in a HeLa cells expression system. The estrogenic potency of 3 beta GSD was also assessed by its capability to induce estrogen-dependent progestin receptors (PR) in the anterior pituitary of castrated female rats. The results demonstrated that 3 beta GSD and 3 alpha GSD were able to activate, in a dose-dependent manner, the hER alpha-mediated transcription of both the beta-galactosidase and the CAT reporter genes in the yeast and HeLa cells expression systems respectively. In both assays the 3 beta derivative of GSD exhibited a significantly greater estrogenic effect than its 3 alpha isomer, while unchanged GSD and 5 alpha GSD were completely ineffective. Neither 3 beta GSD nor 3 alpha GSD exhibited estrogen synergistic actions. Interestingly, the pure steroidal anti-estrogen ICI-182,780 diminished the transactivation induced by 3 beta GSD and 3 alpha GSD in the yeast expression system. Furthermore, administration of 3 beta GSD resulted in a significant increase of estrogen-dependent PR in the anterior pituitaries of castrated rats in comparison with vehicle-treated animals. The characteristics of the 3 beta GSD-induced PR were identical to those induced by estradiol benzoate. The overall results demonstrate that 3 beta GSD and its 3 alpha isomeric alcohol specifically bind to the ER and possess a weak intrinsic estrogenic activity, whereas unmodified GSD does not. The data contribute to a better understanding of the GSD mechanism of action and allow the hypothesis to be advanced that the slight estrogenlike effects attributable to GSD are mediated by its non-phenolic, tetrahydro reduced metabolites.
PMID:10828854 Lemus AE et al; J Endocrinol 165 (3): 693-702 (2000)
Studies on the highly progestogenic compound gestodene have demonstrated that its progestogenic activity in an in-vivo system is far lower than those of its in-vitro binding or receptor activation; however, its androgenic activity in vitro has been confirmed and it appears to have weak binding activity to the glucocorticoid receptor . Its weak estrogenic activity (transactivation of estrogen receptor-mediated gene expression in model cells) appears to derive from its metabolism to the A-ring-reduced metabolites, 3beta- and 3alpha,5alpha-tetrahydrogestodene, and is probably mediated by the activity of 5alpha-reductase. These metabolites appeared to be selective agonists of estrogen receptor-alpha but not of estrogen receptor-beta. The parent compound did not activate estrogen receptors-alpha or -beta.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V91 163 (2007)
For more Mechanism of Action (Complete) data for GESTODENE (6 total), please visit the HSDB record page.


