



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
JP
0
Other Listed Suppliers
0
0
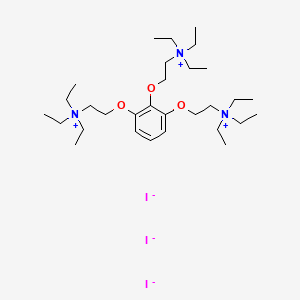

1. Flaxedil
2. Gallamine
3. Gallamine Triethochloride
4. Gallamine Triethyl Iodide
5. Gallamonium Iodide
6. Iodide, Gallamine Triethyl
7. Iodide, Gallamonium
8. Triethiodide, Gallamine
9. Triethochloride, Gallamine
10. Triethyl Iodide, Gallamine
1. 65-29-2
2. Flaxedil
3. Benzcurine Iodide
4. Remyolan
5. Syncurarine
6. Benzkurin
7. Pirolakson
8. Sincurarine
9. Gallaflex
10. Parexyl
11. Pyrolaxon
12. Relaxan
13. Retensin
14. Tricuran
15. Gallamin Triethiodide
16. Gallamone Triethiodide
17. Gallamine Iodide
18. Gallamini Triethiodidum
19. Miowas G
20. Gallamine-3eti
21. Fourneau 2559
22. Gallamine Triiodoethylate
23. Triiodoethylate De Gallamine
24. (v-phenenyltris(oxyethylene))tris(triethylammonium) Triiodide
25. Hl 8583
26. Rp 3697
27. F 2559
28. Gallamoni Jodidum
29. 2-[2,3-bis[2-(triethylazaniumyl)ethoxy]phenoxy]ethyl-triethylazanium;triiodide
30. Gallaminii Iodidum
31. (v-phenenyltris(oxyethylene))tris(triethylammoniumiodide)
32. (v-phenenyltris(oxyethylene))tris(triethylammonium Iodide)
33. Nsc-102690
34. Q3254x40x2
35. Dsstox_cid_3089
36. Gallaminum Triaethjodidum
37. Gallaminum Triaethoiodatum
38. 2,2',2''-(benzene-1,2,3-triyltris(oxy))tris(n,n,n-triethylethan-1-aminium) Iodide
39. Dsstox_rid_76870
40. Dsstox_gsid_23089
41. 3697 R.p.
42. Gallamine Triethiodide [inn]
43. Gallamina Triodoetilato
44. Gallamina Triodoetilato [dcit]
45. Triethiodure De Gallamine
46. 2,2',2''-(benzene-1,2,3-triyltris(oxy))tris(n,n,n-triethylethanaminium) Iodide
47. Gallamini Triethiodidum [inn-latin]
48. Flaxedil (tn)
49. Triiodoethylate De Gallamine [french]
50. Triethiodure De Gallamine [inn-french]
51. Triethioduro De Galamina [inn-spanish]
52. Hsdb 3229
53. Triethioduro De Galamina
54. Cas-65-29-2
55. Sr-01000075317
56. Ncgc00163245-01
57. Einecs 200-605-1
58. Nsc 102690
59. 1,2,3-tris(diethylaminoethoxy)benzene Triethiodide
60. 1,2,3-tris(2-diethylaminoethoxy)benzene Triethiodide
61. 1,2,3-tri(beta-diethylaminoethoxy)benzene Triethiodide
62. Gallamine Triethiodide (usp)
63. 1,2,3-tris(2-triethylammonium Ethoxy)benzene Triiodide
64. 1,2,3-tris(2-diethylaminoethoxy)benzene Tris(ethyliodide)
65. Pyrogallol 1,2,3-(diethylaminoethyl Ether) Trisethyl Iodide
66. Tri(beta-diethylaminoethoxy)-1,2,3-benzene Tri-iodoethylate
67. Triiodoethylate Of Tri(diethylaminoethyloxy)-1,2,3-benzene
68. Unii-q3254x40x2
69. Pyrogallol 1,2,3-(diethylaminoethyl Ether) Tris(ethyliodide)
70. Ammonium, (v-phenenyltris(oxyethylene))tris(triethyl-, Triiodide
71. Gallamine Triethiodide [usp:inn]
72. Triiodure De Tri(beta-triethylammoniumethoxy)-1,2,3 Benzene [french]
73. Tri(iodoethylate) De Tri (beta Diethylaminoethoxy)-1,2,3 Benzene [french]
74. Prestwick_237
75. 2,2',2''-(1,2,3-benzenetriyltris(oxy))tris(n,n,n-triethylethanaminium) Triiodide
76. Ethanaminium, 2,2',2''-(1,2,3-benzenetriyltris(oxy))tris(n,n,n-triethyl)-, Triiodide
77. Ethanaminium, 2,2',2''-(1,2,3-benzenetriyltris(oxy))tris(n,n,n-triethyl-, Triiodide
78. Triiodure De Tri(beta-triethylammoniumethoxy)-1,2,3 Benzene
79. Ammonium, (v-phenenyltris(oxyethylene)tris(triethyl-, Triiodide
80. Tri(iodoethylate) De Tri (beta Diethylaminoethoxy)-1,2,3 Benzene
81. 3.697 R.p.
82. G 8134
83. Ethanaminium, 2,2',2'-(1,2,3-benzenetriyltris(oxy))tris(n,n,n-triethyl)-, Triiodide
84. Chembl1200993
85. Dtxsid5023089
86. Hms502a12
87. Gallamine Triethiodide (flaxedil)
88. Hms1568o16
89. Hms2091h09
90. Hms2095o16
91. Hms3261n21
92. Hms3656g04
93. Hms3712o16
94. Hms3884b21
95. Gallamine Triethiodide [mi]
96. Hy-b0416
97. Tox21_112040
98. Tox21_500550
99. Ccg-40105
100. Gallamine Triethiodide [hsdb]
101. S2471
102. Gallamine Triethiodide [vandf]
103. Akos026749935
104. Gallamine Triethiodide [mart.]
105. Tox21_112040_1
106. Db00483
107. Gallamine Triethiodide [who-dd]
108. Gallamine Triethiodide [who-ip]
109. Lp00550
110. 2,2',2''-[benzene-1,2,3-triyltris(oxy)]tris(n,n,n-triethylethanaminium) Triiodide
111. Ncgc00015482-07
112. Ncgc00093937-01
113. Ncgc00261235-01
114. As-57694
115. Gallamine Triethiodide [orange Book]
116. Eu-0100550
117. Ft-0703297
118. G0554
119. Gallamine Triethiodide [usp Impurity]
120. Sw196544-3
121. Gallamini Triethiodidum [who-ip Latin]
122. C76041
123. D02292
124. Q3094785
125. Sr-01000075317-1
126. Sr-01000075317-3
127. Sr-01000075317-6
128. W-104798
129. Gallamine Triethiodide, European Pharmacopoeia (ep) Reference Standard
130. Gallamine Triethiodide, United States Pharmacopeia (usp) Reference Standard
131. (2-{2,3-bis[2-(triethylazaniumyl)ethoxy]phenoxy}ethyl)triethylazanium Triiodide
132. 6b,7a-dihydro-7h-cycloprop[a]acenaphthylene-7-carboxylicacidethylester
133. Gallamine Triethiodide, >=98% (tlc), Powder, Muscarinic Receptor Antagonist
134. Ethanaminium, 2,2',2''-(benzene-1,2,3-triyltris(oxy))tris(n,n,n-triethyl-, Triiodide
| Molecular Weight | 891.5 g/mol |
|---|---|
| Molecular Formula | C30H60I3N3O3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 21 |
| Exact Mass | 891.1769 g/mol |
| Monoisotopic Mass | 891.1769 g/mol |
| Topological Polar Surface Area | 27.7 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 489 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 4 |
Neuromuscular Nondepolarizing Agents; Nicotinic Antagonists
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A NEUROMUSCULAR BLOCKING DRUG SIMILAR IN ITS ACTIONS & USES TO TUBOCURARINE CHLORIDE. ...IN GENERAL, IT HAS VERY LITTLE ACTION ON AUTONOMIC GANGLIA, BUT IT USUALLY BLOCKS CARDIAC VAGUS... IT ALSO DOES NOT RELEASE HISTAMINE /IN LOW DOSES/.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 852
... IT HAS NO PERCEPTIBLE EFFECT ON NEWBORN INFANTS WHEN USUAL DOSES ARE GIVEN FOR CESAREAN SECTION & VAGINAL DELIVERY & TONE OF UTERUS IS NOT AFFECTED.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 337
...HAS BEEN REPORTED TO REDUCE OCULAR PRESSURE SLIGHTLY IN PT.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 519
For more Therapeutic Uses (Complete) data for GALLAMINE TRIETHIODIDE (9 total), please visit the HSDB record page.
THE NEUROMUSCULAR BLOCKING AGENTS ARE POTENTIALLY HAZARDOUS DRUGS. ... THEY SHOULD BE ADMINISTERED TO PATIENTS ONLY BY ANESTHESIOLOGISTS & OTHER CLINICIANS WHO HAVE HAD EXTENSIVE TRAINING IN THEIR USE & IN A SETTING WHERE FACILITIES FOR RESPIRATORY & CARDIOVASCULAR RESUSCITATION ARE IMMEDIATELY AT HAND. /NEUROMUSCULAR BLOCKING AGENTS/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 190
IT SHOULD BE USED CAUTIOUSLY IF TACHYCARDIA PREEXISTS. .../SINCE IT/ IS ELIMINATED MAINLY BY RENAL EXCRETION...ITS ACTION MAY BE PROLONGED IF THERE IS RENAL DYSFUNCTION.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 863
... /IT SHOULD NOT/ BE USED IN PT WITH RENAL DISEASE.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 338
CROSS SENSITIVITY BETWEEN ALCURONIUM & D-TUBOCURARINE OCCURS & SIMILAR SITUATION MAY EXIST FOR SUXAMETHONIUM & GALLAMINE.
PMID:7396189 FISHER MM; ANAESTH INTENSIVE CARE 8 (2): 211 (1980)
For more Drug Warnings (Complete) data for GALLAMINE TRIETHIODIDE (6 total), please visit the HSDB record page.
For use as adjuncts to anesthesia to induce skeletal muscle relaxation and to facilitate the management of patients undergoing mechanical ventilation
Gallamine Triethiodide is a nondepolarizing neuromuscular blocking drug (NDMRD) used as an adjunct to anesthesia to induce skeletal muscle relaxation. The actions of gallamine triethiodide are similar to those of tubocurarine, but this agent blocks the cardiac vagus and may cause sinus tachycardia and, occasionally, hypertension and increased cardiac output. Muscle groups differ in their sensitivity to these types of relaxants with ocular muscles (controlling eyelids) being most sensitive, followed by the muscles of the neck, jaw, limbs and then abdomen. The diaphragm is the least sensitive muscle to NDMRDs. Although the nondepolarizing neuromuscular blocking drugs do not have the same adverse effects as succinylcholine, their onset of action is slower. They also have a longer duration of action, making them more suitable for maintaining neuromuscular relaxation during major surgical procedures.
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
Nicotinic Antagonists
Drugs that bind to nicotinic cholinergic receptors (RECEPTORS, NICOTINIC) and block the actions of acetylcholine or cholinergic agonists. Nicotinic antagonists block synaptic transmission at autonomic ganglia, the skeletal neuromuscular junction, and at central nervous system nicotinic synapses. (See all compounds classified as Nicotinic Antagonists.)
STUDIES HAVE DEMONSTRATED THAT GALLAMINE IS EXCRETED IN DOG URINE AT RATE FASTER THAN OTHER MUSCLE RELAXANTS. .../IT/ DID NOT CROSS BLOOD-CEREBROSPINAL FLUID BARRIER. OTHER STUDIES...HAVE DETECTED GALLAMINE IN CEREBROSPINAL FLUID IN CONCN APPROACHING THOSE IN PLASMA DURING 1ST HR AFTER IV INJECTION. /GALLAMINE/
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 156
GALLAMINE IS ELIMINATED MAINLY BY RENAL EXCRETION...
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 863
... GALLAMINE CROSSES PLACENTAL BARRIER ...
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 337
TRACE AMT OF GALLAMINE APPEARS IN FETUS 3 MIN AFTER ADMIN. /GALLAMINE, FROM TABLE/
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 101
For more Absorption, Distribution and Excretion (Complete) data for GALLAMINE TRIETHIODIDE (6 total), please visit the HSDB record page.
.../GALLAMINE/ IS UNMETABOLIZED. /GALLAMINE/
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 156
135 minutes /From table/
Young, L.Y., M.A. Koda-Kimble (eds.). Applied Therapeutics. The Clinical Use of Drugs. 6th ed. Vancouver, WA., Applied Therapeutics, Inc. 1995., p. 8-11
It competes with acetylcholine (ACh) molecules and binds to muscarinic acetylcholine receptors on the post-synaptic membrane of the motor endplate. It acts by combining with the cholinergic receptor sites in muscle and competitively blocking the transmitter action of acetylcholine. It blocks the action of ACh and prevents activation of the muscle contraction process. It can also act on nicotinic presynaptic acetylcholine receptors which inhibits the release of ACh.
GALLAMINE TRIETHIODIDE...PRODUCES SKELETAL MUSCLE RELAXATION BY COMBINING WITH RECEPTOR SITE AT NEUROMUSCULAR JUNCTION & BLOCKING ACTION OF NEUROTRANSMITTER ACETYLCHOLINE.
Evaluations of Drug Interactions. 2nd ed. and supplements. Washington, DC: American Pharmaceutical Assn., 1976, 1978., p. 75
IN RAT PHRENIC NERVE-DIAPHRAGM GALLAMINE HAD NO SIGNIFICANT EFFECTS ON ELECTROGENIC PROPERTIES OF EXCITABLE MEMBRANES OF MOTOR NERVE TERMINALS & MUSCLE FIBERS; IT DEPRESSED RESPONSE OF POSTSYNAPTIC RECEPTORS TO ACTION OF ACETYLCHOLINE.
GALINDO A, KELLY PJ; MECHANISM OF ACTION OF GALLAMINE; ANESTH ANALG (CLEVELAND) 59(7) 484 (1980)
AT NEUROMUSCULAR JUNCTIONS IN MICE AND FROGS, FOLLOWING STEP CHANGES OF MEMBRANE POTENTIAL FROM -70 TO -130 MV, GALLAMINE (5 UMOL) IN THE PRESENCE OF ACETYLCHOLINE (3 UMOL) CAUSED AN INITIAL RAPID DECR IN CURRENT FOLLOWED BY OPENING OF CHANNELS AT A SLOWER RATE THAN WITH ACETYLCHOLINE ALONE. WHEN THE INTERNAL POTENTIAL WAS REDUCED TO -70 MV, THERE WAS A RAPID INCR IN CURRENT AT FIRST, FOLLOWED BY THE USUAL DECR WHICH WAS AGAIN SLOWER THAN NORMAL. THUS, GALLAMINE MAY PRODUCE A POTENTIAL-DEPENDENT BLOCK OF OPEN ION CHANNELS.
COLQUHOUN D, SHERIDAN RE; MODES OF ACTION OF GALLAMINE AT THE NEUROMUSCULAR JUNCTION; BR J PHARMACOL 66(1) 78 (1979)


