
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book

0
Europe

0
Canada

0
Australia

0
South Africa

0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
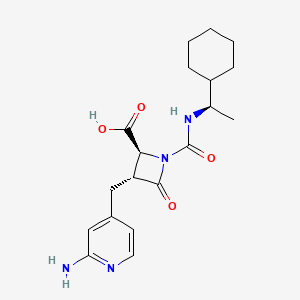

1. Ep-7041
1. Ep-7041
2. 1803270-60-1
3. Bke5cvp3fs
4. (2s,3r)-3-((2-aminopyridin-4-yl)methyl)-1-(((1r)-1-cyclohexylethyl)carbamoyl)-4-oxoazetidine-2-carboxylic Acid
5. (2s,3r)-3-[(2-aminopyridin-4-yl)methyl]-1-[[(1r)-1-cyclohexylethyl]carbamoyl]-4-oxoazetidine-2-carboxylic Acid
6. (2s,3r)-3-[(2-aminopyridin-4-yl)methyl]-1-{[(1r)-1-cyclohexylethyl]carbamoyl}-4-oxoazetidine-2-carboxylic Acid
7. Refchem:137078
8. Frunexian (usan)
9. Frunexian [usan]
10. Frunexian [inn]
11. Orb2277627
12. Chembl5314583
13. Schembl16997955
14. Gtpl11990
15. Ep 7041 [who-dd]
16. Bdbm50621716
17. Ep7041
18. Da-73563
19. Hy-156613
20. Cs-0886205
21. D12563
22. 2-azetidinecarboxylic Acid, 3-[(2-amino-4-pyridinyl)methyl]-1-[[[(1r)-1-cyclohexylethyl]amino]carbonyl]-4-oxo-, (2s,3r)-
| Molecular Weight | 374.4 g/mol |
|---|---|
| Molecular Formula | C19H26N4O4 |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 126 |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 578 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
ABOUT THIS PAGE

