



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
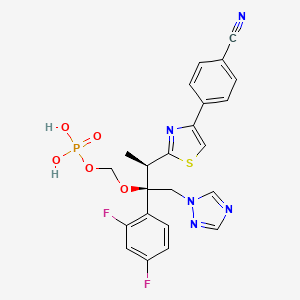

1. Bms-379224
1. 351227-64-0
2. Fosravuconazole [inn]
3. Bms-379224
4. Ravuconazole Phosphoester
5. Ravuconazole Methylphosphate
6. E1224 Free Acid
7. Ravuconazole Methyl Phosphate
8. E-1224 Free Acid
9. Chebi:34549
10. L4q6o5430l
11. Bms 379224
12. (((2r,3r)-3-(4-(4-cyanophenyl)thiazol-2-yl)-2-(2,4-difluorophenyl)-1-(1h-1,2,4-triazol-1-yl)butan-2-yl)oxy)methyl Dihydrogen Phosphate
13. Benzonitrile, 4-(2-((1r,2r)-2-(2,4-difluorophenyl)-1-methyl-2-((phosphonooxy)methoxy)-3-(1h-1,2,4-triazol-1-yl)propyl)-4-thiazolyl)-
14. Unii-l4q6o5430l
15. Fosravuconazole [mi]
16. Chembl333325
17. Schembl1434200
18. Fosravuconazole [who-dd]
19. Dtxsid70188627
20. Db15204
21. [(2r,3r)-3-[4-(4-cyanophenyl)-1,3-thiazol-2-yl]-2-(2,4-difluorophenyl)-1-(1,2,4-triazol-1-yl)butan-2-yl]oxymethyl Dihydrogen Phosphate
22. Hy-16779
23. Cs-0012407
24. Q27116141
25. 4-[2-[(1r,2r)-1-methyl-2-(phosphonooxymethoxy)-2-(2,4-difluorophenyl)-3-(1h-1,2,4-triazole-1-yl)propyl]thiazole-4-yl]benzonitrile
| Molecular Weight | 547.5 g/mol |
|---|---|
| Molecular Formula | C23H20F2N5O5PS |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 10 |
| Exact Mass | 547.08908325 g/mol |
| Monoisotopic Mass | 547.08908325 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 37 |
| Formal Charge | 0 |
| Complexity | 859 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
D - Dermatologicals
D01 - Antifungals for dermatological use
D01B - Antifungals for systemic use
D01BA - Antifungals for systemic use
D01BA03 - Fosravuconazole


