


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
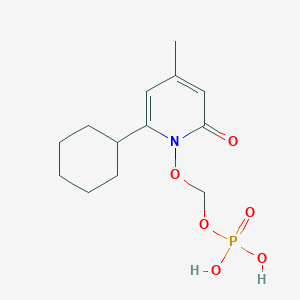

1. Ciclopirox-pom
2. Fosciclopirox [inn]
3. 1380539-06-9
4. G8fr269qpq
5. 2(1h)-pyridinone, 6-cyclohexyl-4-methyl-1-((phosphonooxy)methoxy)-
6. 6-cyclohexyl-4-methyl-1-((phosphonooxy)methoxy)-2(1h)-pyridinone
7. (2-cyclohexyl-4-methyl-6-oxopyridin-1-yl)oxymethyl Dihydrogen Phosphate
8. ((6-cyclohexyl-4-methyl-2-oxopyridin-1(2h)-yl)oxy)methyl Dihydrogen Phosphate
9. Unii-g8fr269qpq
10. Fosciclopirox [who-dd]
11. Schembl9103427
12. Chembl4802149
13. Ex-a5502
14. Hy-109174
15. Cs-0116368
| Molecular Weight | 317.27 g/mol |
|---|---|
| Molecular Formula | C13H20NO6P |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 5 |
| Exact Mass | 317.10282436 g/mol |
| Monoisotopic Mass | 317.10282436 g/mol |
| Topological Polar Surface Area | 96.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 503 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


