



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
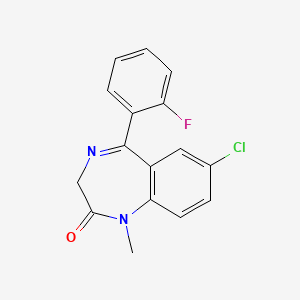

1. 1-methyl-5-(2-fluorophenyl)-7-chloro-1,3-dihydro-2h-(1,4)benzodiazepin-2-one
2. Fludiazepan
3. Id 540
1. Erispan
2. 3900-31-0
3. Fludiazepamum [inn-latin]
4. 7-chloro-5-(o-fluorophenyl)-1,3-dihydro-1-methyl-2h-1,4-benzodiazepin-2-one
5. Id-540
6. 7-chloro-5-(2-fluorophenyl)-1-methyl-3h-1,4-benzodiazepin-2-one
7. Chebi:31618
8. 7f64a2k16z
9. Fludiazepamum
10. Fludiazepam [inn:jan]
11. Id 540
12. Erispan (tn)
13. Brn 0686115
14. Unii-7f64a2k16z
15. Dea No. 2759
16. 7-chloro-5-(2-fluorophenyl)-1-methyl-1h-1,4-benzodiazepin-2(3h)-one
17. 7-chloro-5-(2-fluorophenyl)-1,3-dihydro-1-methyl-2h-1,4-benzodiazepin-2-one
18. 7-chloro-5-(2-fluorophenyl)-1-methyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one
19. Fludiazepam [mi]
20. Fludiazepam [inn]
21. Fludiazepam [jan]
22. Fludiazepam (jp17/inn)
23. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(2-fluorophenyl)-1,3-dihydro-1-methyl-
24. 2h-1,4-benzodiazepin-2-one, 7-chloro-5-(o-fluorophenyl)-1,3-dihydro-1-methyl-
25. Fludiazepam [mart.]
26. Fludiazepam [who-dd]
27. 5-24-04-00320 (beilstein Handbook Reference)
28. Chembl13291
29. Schembl156039
30. Dtxsid00192277
31. Db01567
32. Ro-5-3438
33. 2h-1,4-benzodiazepin-2-one, 1,3-dihydro-7-chloro-5-(o-fluorophenyl)-1-methyl-
34. D01354
35. 900f310
36. Q3486981
37. 7-chloro-5-(2-fluorophenyl)-1-methyl-1,3-dihydro-2h-1,4-benzodiazepin-2-one #
38. 7-chloro-5-(2-fluorophenyl)-1-methyl-2,3-dihydro-1h-1,4-benzodiazepin-2-one
| Molecular Weight | 302.73 g/mol |
|---|---|
| Molecular Formula | C16H12ClFN2O |
| XLogP3 | 2.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 302.0622189 g/mol |
| Monoisotopic Mass | 302.0622189 g/mol |
| Topological Polar Surface Area | 32.7 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 442 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used for the short-term treatment of anxiety disorders.
Fludiazepam is a benzodiazepine derivative. It possesses anxiolytic, anticonvulsant, sedative and skeletal muscle relaxant properties. Fludiazepam accumulates primarily in the cortex and thalamus.
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
Muscle Relaxants, Central
A heterogeneous group of drugs used to produce muscle relaxation, excepting the neuromuscular blocking agents. They have their primary clinical and therapeutic uses in the treatment of muscle spasm and immobility associated with strains, sprains, and injuries of the back and, to a lesser degree, injuries to the neck. They have been used also for the treatment of a variety of clinical conditions that have in common only the presence of skeletal muscle hyperactivity, for example, the muscle spasms that can occur in MULTIPLE SCLEROSIS. (From Smith and Reynard, Textbook of Pharmacology, 1991, p358) (See all compounds classified as Muscle Relaxants, Central.)
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA17 - Fludiazepam
Hepatic.
Fludiazepam has similar action to diazepam, but binds with four times more affinity to benzodiazepine receptors than diazepam.


