
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
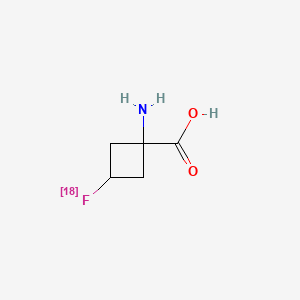

1. (18f)ge-148
2. (1r,3r)-1-amino-3(18f)fluorocyclobutane-1-carboxylic Acid
3. 1-amino-3-fluorocyclobutane-1-carboxylic Acid
4. Anti-(18f)facbc
5. Axumin
6. Cyclobutanecarboxylic Acid, 1-amino-3-(fluoro-18f)-, Trans-
7. F(18)-facbc
8. F(18)1-amino-3-fluorocyclobutane-1-carboxylic Acid
9. F-facbc
10. Fluciclovine F 18
11. Fluciclovine F-18
12. Ge-148 (18f)
13. Ge-148 F-18
14. Nmk 36
15. Nmk-36
16. Nmk36 Cpd
1. Fluciclovine F-18
2. Axumin
3. 222727-39-1
4. Fluciclovine F 18
5. Facbc
6. Fluciclovine F18
7. Nmk36
8. Ge-148
9. (18f)fluciclovine
10. Fluciclovine (18f) [inn]
11. Nmk-36
12. (18f)ge-148
13. Ge-148 F-18
14. Ge-148 (18f)
15. (18f)facbc
16. Facbc F-18
17. 38r1q0l1ze
18. Anti-1-amino-3-(18f)fluorocyclobutane-1-carboxylic Acid
19. (1r,3r)-1-amino-3(18f)fluorocyclobutane-1-carboxylic Acid
20. [18f]fluciclovine
21. [18f]facbc
22. Fluciclovine (18f) (inn)
23. 1-amino-3-(18f)fluoranylcyclobutane-1-carboxylic Acid
24. Cyclobutanecarboxylic Acid, 1-amino-3-(fluoro-18f)-, Trans-
25. Anti-(18f)fabc
26. Fluciclovine F18 [usan]
27. Anti-1-amino-3-[18f]fluorocyclobutane-1-carboxylic Acid
28. Unii-38r1q0l1ze
29. Fluciclovine ((sup 18)f)
30. Fluciclovine-f18
31. Moli001120
32. Fluciclovine ((sup 18)f) [inn]
33. Axumin (tn)
34. Nmk36 Cpd
35. Anti-[18f]facbc
36. Syn-3-[18f]facbc
37. Fluciclovine F-18 (usan)
38. Chembl254468
39. Chembl447701
40. Nmk 36
41. Schembl10017245
42. Schembl11939897
43. Schembl11939900
44. Chebi:134703
45. Dtxsid601027796
46. Fluciclovine (18f) [mi]
47. Fluciclovine F 18 [usan]
48. Bcp24384
49. Axumincn Facbccn Fluciclovine (18f)cn Ge 148cn Nmk 36
50. At31246
51. Db13146
52. Fluciclovine (18f) [who-dd]
53. Fluciclovine F-18 [orange Book]
54. D10860
55. F(18)1-amino-3-fluorocyclobutane-1-carboxylic Acid
56. Q25313613
57. Syn-1-amino-3-[18f]fluorocyclobutane-1-carboxylic Acid
58. Anti-3[18f] Facbc;f18; Anti-1-amino-3-18f-fluorocyclobutane-1-carboxylic Acid (facbc)
| Molecular Weight | 132.12 g/mol |
|---|---|
| Molecular Formula | C5H8FNO2 |
| XLogP3 | -2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 132.056441 g/mol |
| Monoisotopic Mass | 132.056441 g/mol |
| Topological Polar Surface Area | 63.3 Ų |
| Heavy Atom Count | 9 |
| Formal Charge | 0 |
| Complexity | 142 |
| Isotope Atom Count | 1 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fluciclovine is indicated as a detection agent for positron emission tomography (PET) in men with suspected prostate cancer recurrence based on elevated blood prostate specific antigen (PSA) levels following prior treatment. The overexpression of L-type amino acid transporters such as LAT1 and LAT3 that mediate the uptake of essential amino acids has been extensively reported as a tumoral mechanism of cell growth.
FDA Label
This medicinal product is for diagnostic use only.
Axumin is indicated for Positron Emission Tomography (PET) imaging to detect recurrence of prostate cancer in adult men with a suspected recurrence based on elevated blood prostate specific antigen (PSA) levels after primary curative treatment.
Following intravenous administration, the tumor-to-normal tissue contrast is highest between 2 and 10 minutes after injection, with a 63% reduction in mean tumor uptake at 90 minutes after injection. The scanning time point should be evaluated carefully as an early scanning can present an increased blood pool and a late scanning will translate into an increased muscle uptake. These variations should always be considered in the image interpretation.
V09IX12
V - Various
V09 - Diagnostic radiopharmaceuticals
V09I - Tumour detection
V09IX - Other diagnostic radiopharmaceuticals for tumour detection
V09IX12 - Fluciclovine (18F)
Absorption
After intravenous administration of fluciclovine, the major distribution happens in liver (14%), red bone marrow (12%), lung (7%), myocardium (4%) and pancreas (3%). With increasing time, the dose gets distributed into skeletal muscle.
Route of Elimination
In the first four hours post-injection, 3% of administered dose is excreted in the urine which increases to 5% after 24 hours post-injection.
Volume of Distribution
The compartmental volume of distribution of fluciclovine is in prostate 0.97 L, vesicle 0.79 L, red bone marrow 0.98 L, gluteus muscle 2.13 L and obturator muscle 2.23 L.
Clearance
Fluciclovine renal clearance and excretion is minimal.
Fluciclovine is not metabolized and it is not incorporated into newly synthesized proteins.
Fluciclovine is a cyclotron produced radionuclide that decays by positron emission (+ decay, 96.7%) and orbital electron capture (3.3%) to stable oxygen 18 with a physical half-life of 109.7 minutes.
Fluciclovine is transported into the prostate cancer cells via ASCT2 and LAT1 transporters. The activity of LAT1 gets increased in acidic pH, condition that is developed intra-tumorally at certain size. The uptake of fluciclovine presents an androgen-dependent dynamic in hormone sensitive cells.
Related Excipient Companies

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
79
PharmaCompass offers a list of UNII-38R1Q0L1ZE API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right UNII-38R1Q0L1ZE manufacturer or UNII-38R1Q0L1ZE supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred UNII-38R1Q0L1ZE manufacturer or UNII-38R1Q0L1ZE supplier.
PharmaCompass also assists you with knowing the UNII-38R1Q0L1ZE API Price utilized in the formulation of products. UNII-38R1Q0L1ZE API Price is not always fixed or binding as the UNII-38R1Q0L1ZE Price is obtained through a variety of data sources. The UNII-38R1Q0L1ZE Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Fluciclovine F 18 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Fluciclovine F 18, including repackagers and relabelers. The FDA regulates Fluciclovine F 18 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Fluciclovine F 18 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Fluciclovine F 18 supplier is an individual or a company that provides Fluciclovine F 18 active pharmaceutical ingredient (API) or Fluciclovine F 18 finished formulations upon request. The Fluciclovine F 18 suppliers may include Fluciclovine F 18 API manufacturers, exporters, distributors and traders.
Fluciclovine F 18 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Fluciclovine F 18 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Fluciclovine F 18 GMP manufacturer or Fluciclovine F 18 GMP API supplier for your needs.
A Fluciclovine F 18 CoA (Certificate of Analysis) is a formal document that attests to Fluciclovine F 18's compliance with Fluciclovine F 18 specifications and serves as a tool for batch-level quality control.
Fluciclovine F 18 CoA mostly includes findings from lab analyses of a specific batch. For each Fluciclovine F 18 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Fluciclovine F 18 may be tested according to a variety of international standards, such as European Pharmacopoeia (Fluciclovine F 18 EP), Fluciclovine F 18 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Fluciclovine F 18 USP).

