



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
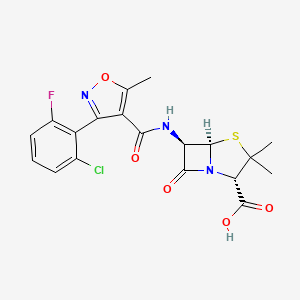

1. Floxacillin
2. Fluorochloroxacillin
1. Floxacillin
2. 5250-39-5
3. Floxapen
4. Flucloxacilina
5. Flucloxacilline
6. Flucloxacillinum
7. Floxacillin [usan]
8. Flucloxacillin Sodium
9. Flucloxacilina [inn-spanish]
10. Flucloxacilline [inn-french]
11. Flucloxacillinum [inn-latin]
12. Brl 2039
13. 3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolylpenicillin
14. Flucloxacillin-sodium
15. Chebi:5098
16. Floxacillin (usan)
17. Flucloxacillin (inn)
18. Flucloxacillin [inn]
19. Brl-2039
20. Floxapen (tn)
21. (2s,5r,6r)-6-[[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
22. 43b2m34g2v
23. (2s,5r,6r)-6-({[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
24. 6-(3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolecarboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid
25. Floxacillin Sodium Anhydrous
26. Flucloxacillin Sodium Anhydrous
27. 4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid,6-[[[3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl]carbonyl]amino]-3,3-dimethyl-7-oxo-,(2s,5r,6r)-
28. Mfipc
29. Nsc-277175
30. Einecs 226-051-0
31. Unii-43b2m34g2v
32. Flucloxacillin,(s)
33. Floxacillin [mi]
34. Epitope Id:117416
35. Schembl3823
36. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2s(2alpha,5alpha,6beta))
37. Flucloxacillin [mart.]
38. Chembl222645
39. Flucloxacillin [who-dd]
40. Dtxsid8023056
41. Gtpl10910
42. Brl2039
43. Hy-a0246
44. Zinc4102187
45. Bdbm50370590
46. Db00301
47. Ncgc00485420-01
48. (2s,5r,6r)-6-({[3-(2-chloro-6-fluorophenyl)-5-methylisoxazol-4-yl]carbonyl}amino)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
49. (2s,5r,6r)-6-[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-amido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
50. 6beta-[3-(2-chloro-6-fluorophenyl)-5-methyl-1,2-oxazole-4-carboxamido]-2,2-dimethylpenam-3alpha-carboxylic Acid
51. Nci60_002254
52. Cs-0017591
53. D04196
54. 250f395
55. Q1994556
56. W-105817
57. Brd-k27871792-001-01-8
58. Flucloxacillin, Antibiotic For Culture Media Use Only
59. (2s,5r,6r)-6-(3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic Acid
60. 4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic Acid, 6-(((3-(2-chloro-6-fluorophenyl)-5-methyl-4-isoxazolyl)carbonyl)amino)-3,3-dimethyl-7-oxo-, (2s(2.alpha.,5.alpha.,6.beta.))
| Molecular Weight | 453.9 g/mol |
|---|---|
| Molecular Formula | C19H17ClFN3O5S |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 4 |
| Exact Mass | 453.0561477 g/mol |
| Monoisotopic Mass | 453.0561477 g/mol |
| Topological Polar Surface Area | 138 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 758 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 3 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Used to treat bacterial infection by susceptible microorganisms.
Flucloxacillin is a penicillin beta-lactam antibiotic used in the treatment of bacterial infections caused by susceptible, usually gram-positive, organisms. The name "penicillin" can either refer to several variants of penicillin available, or to the group of antibiotics derived from the penicillins. Flucloxacillin has in vitro activity against gram-positive and gram-negative aerobic and anaerobic bacteria. The bactericidal activity of Flucloxacillin results from the inhibition of cell wall synthesis and is mediated through flucloxacillin binding to penicillin binding proteins (PBPs). Flucloxacillin is stable against hydrolysis by a variety of beta-lactamases, including penicillinases, and cephalosporinases and extended spectrum beta-lactamases.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01CF05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01C - Beta-lactam antibacterials, penicillins
J01CF - Beta-lactamase resistant penicillins
J01CF05 - Flucloxacillin
Absorption
Bioavailability is 5070% following oral administration.
Hepatic.
Flucloxacillin has known human metabolites that include 6-[[3-(2-Chloro-6-fluorophenyl)-5-(hydroxymethyl)-1,2-oxazole-4-carbonyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
0.751 hour
By binding to specific penicillin-binding proteins (PBPs) located inside the bacterial cell wall, flucloxacillin inhibits the third and last stage of bacterial cell wall synthesis. Cell lysis is then mediated by bacterial cell wall autolytic enzymes such as autolysins; it is possible that flucloxacillin interferes with an autolysin inhibitor.


