



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
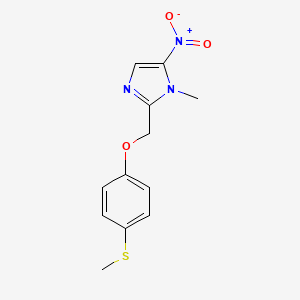

1. Hoe 239
1. 59729-37-2
2. 1-methyl-2-((4-(methylthio)phenoxy)methyl)-5-nitro-1h-imidazole
3. Hoe-239
4. Hoe 239
5. 1-methyl-2-[(4-methylsulfanylphenoxy)methyl]-5-nitroimidazole
6. 306erl82ir
7. 1-methyl-2-((p-(methylthio)phenoxy)methyl)-5-nitroimidazole
8. 1-methyl-2-((4-(methylthio)phenoxy)-methyl)-5-nitro-1h-imidazole
9. Fexinidazol
10. Fexinidazolum
11. Fexinidazole [inn]
12. Fexinidazol [inn-spanish]
13. Fexinidazolum [inn-latin]
14. Unii-306erl82ir
15. Fexinidazole (usan/inn)
16. Fexinidazole [usan:inn]
17. Fexinidazole [usan]
18. Fexinidazole (hoe-239)
19. Mls006010234
20. Fexinidazole [who-dd]
21. Schembl1163575
22. Zinc1448
23. Chembl1631694
24. Dtxsid00208448
25. 1-methyl-2-(4-methylthiophenyl-oxymethyl)-5-nitro-imidazole
26. Fexinidazole [orange Book]
27. Bcp07460
28. Who 4142
29. Mfcd00866607
30. S2600
31. Akos016010427
32. Cs-5535
33. Db12265
34. Sb16940
35. Hy-13801
36. Smr004701311
37. D11252
38. A869137
39. Q5446115
40. 1-methyl-2-(4-methylthiophenyloxymethyl)-5-nitro-imidazole
41. 1h-imidazole, 1-methyl-2-((4-(methylthio)phenoxy)methyl)-5-nitro-
| Molecular Weight | 279.32 g/mol |
|---|---|
| Molecular Formula | C12H13N3O3S |
| XLogP3 | 2.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 279.06776246 g/mol |
| Monoisotopic Mass | 279.06776246 g/mol |
| Topological Polar Surface Area | 98.2 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 305 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Fexinidazole is a nitroimidazole indicated for the treatment of both first-stage (hemolymphatic) and second-stage (meningoencephalitic) _Trypanosoma brucei gambiense_ human African trypanosomiasis (HAT) in patients 6 years of age and older weighing at least 20 kg. Due to the decreased efficacy observed in patients with severe second stage HAT (cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/L), fexinidazole should only be used in these patients if there are no other available treatment options.
Fexinidazole is a 2-substituted 5-nitroimidazole that is likely activated by parasitic nitroreductases to highly reactive species, leading to DNA and protein damage and eventual parasite death. The dosing schedule is designed to ensure a high enough concentration of fexinidazole and its reactive metabolites for at least 48 hours, which from _in vitro_ studies was shown to be the minimum exposure time that was effectively trypanocidal. Although fexinidazole is effective in late-stage _T. brucei gambiense_ HAT, it is less effective than NECT therapy in patients with severe (cerebrospinal fluid white blood cell count (CSF-WBC) >100 cells/L at baseline) disease. It should only be used in these patients if there are no other available treatment options. Fexinidazole has been shown to prolong the QT interval in a dose-dependent manner and was also associated with a higher incidence of insomnia, headache, tremors, psychiatric disorders, and suicidal ideation in clinical trials; patients with pre-existing conditions or concomitant medications that could aggravate any of these effects should be treated with caution. In addition, fexinidazole has been associated with neutropenia and elevations in liver transaminases, which should be monitored. Nitroimidazoles like fexinidazole have been associated with a disulfiram-like reaction when used concomitantly with alcohol and psychotic reactions when taken with [disulfiram] itself; patients should avoid alcohol and [disulfiram] when taking fexinidazole.
P - Antiparasitic products, insecticides and repellents
P01 - Antiprotozoals
P01C - Agents against leishmaniasis and trypanosomiasis
P01CA - Nitroimidazole derivatives
P01CA03 - Fexinidazole
Absorption
Fexinidazole is well absorbed, although the rate and extent of absorption are less than dose-proportional; after a 14-day administration schedule, the mean Cmax and AUClast increased by 1.17 and 1.34, or by 1.5 and 1.61, when the dose was either doubled or tripled. Following absorption, fexinidazole is rapidly converted to its M1 metabolite, which undergoes a slower transformation to M2 over time. This is reflected in the Tmax of fexinidazole, M1, and M2 as 4 (0-9), 4 (0-6), and 6 (0-24) hours, respectively. In healthy adults given an 1800 mg loading dose followed by 1200 mg daily over 14 days, the mean Cmax for fexinidazole was 1.6 0.4 g/mL on day 1, 0.8 0.3 g/mL on day 2, and 0.5 0.2 g/mL on day 3. The relevant values for M1 were 8.1 2.2, 8.0 2.3, and 5.9 2.1, while for M2 they were 7.5 3.3, 19.6 5.4, and 12.5 3.5 g/mL. Similarly, the AUC for fexinidazole was 14.3 2.6, 11.6 2.2, and 7.0 2.5, for M1 was 102.3 28.5, 127.9 49.2, and 84.2 36.3, and for M2 was 110.1 41.1, 391.5 126.7, and 252.4 73.6 g\*h/mL. Concomitant food intake increases the Cmax and AUC of fexinidazole, M1, and M2 by 2-5 fold without significantly changing the metabolite ratios. There are no clear effects of age, renal, or hepatic impairment on absorption or plasma parameters of fexinidazole or its metabolites; further studies may be required to confirm/refute these observations.
Route of Elimination
Elimination is almost entirely extra-renal; roughly 0.75-3.15% of a fexinidazole dose was recovered in urine over 168 h, primarily as M1 and M2 metabolites.
Volume of Distribution
Fexinidazole has an apparent volume of distribution of 3222 1199 L.
Clearance
Fexinidazole has a mean apparent day 4 clearance of 161 37 L/h.
Fexinidazole is metabolized by a variety of enzymes including the CYP450 enzymes CYP1A2, 2B6, 2C19, 2D6, 3A4, and 3A5 as well as flavin mono-oxygenase-3 (FMO-3). Fexinidazole is first transformed to the sulfoxide M1 and then the sulfone M2, which does not appear to undergo further metabolism.
Fexinidazole, M1, and M2 have mean day 10 half-lives of 15 6, 16 6, and 23 4 hours, respectively.
Human African trypanosomiasis (HAT) is caused by two subspecies of _Trypanosoma brucei_, _T. brucei gambiense_ and _T. brucei rhodesiense_, with _T. brucei gambiense_ HAT accounting for ~97% of the total disease burden. Transmitted by the bite of an infected tsetse fly, HAT begins as a local infection at the bite site before disseminating throughout the blood and reticuloendothelial system (first or hemolymphatic stage) and eventually crossing the blood-brain barrier (second or meningoencephalitic stage). First stage _T. brucei gambiense_ HAT is characterized by fever, headache, swollen lymph nodes, pruritus, and other non-specific symptoms. Progression to the second stage results in progressive deterioration of neurological function, including sleep disturbances (HAT is also referred to as sleeping sickness), tremors, ataxia, abnormal behaviour, confusion, and coma; myocarditis and endocrine hypothalamic-hypophyseal dysfunction may also be present. If left untreated, HAT is fatal. Fexinidazole is the first all-oral treatment for _T. brucei gambiense_ HAT. Both fexinidazole and its two main metabolites, a sulfoxide (M1) and sulfone (M2) metabolite, possess _in vitro_ activity against _T. brucei gambiense_, _T. brucei rhodesiense_, and _T. brucei brucei_ in the 0.2-0.9 g/mL range. Further studies revealed _in vivo_ efficacy in HAT animal models and acceptable toxicity profiles, both in animal and human subjects. Crucially, fexinidazole was shown to be non-inferior to existing [nifurtimox]/[eflornithine] combination therapy (NECT) in late-stage _T. brucei gambiense_ infection. The precise mechanism of action of fexinidazole remains unknown. However, it is suggested that bacterial-like nitroreductases encoded by trypanosomes activate fexinidazole and its M1/M2 metabolites through reduction to form reactive intermediates capable of damaging DNA and proteins. Whole-body autoradiography of [14C]-labelled fexinidazole in rats revealed broad distribution into all tissues, including an observed brain-to-blood concentration ratio of 0.4-0.6. Therefore, fexinidazole is capable of direct toxicity against trypanosomes throughout the body and in the brain, which is consistent with its efficacy against both early and late-stage infections.


