


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
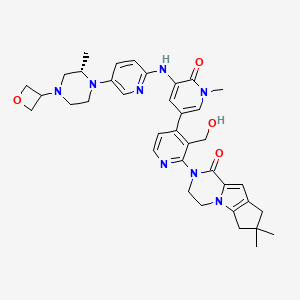

1. 2-(3'-(hydroxymethyl)-1-methyl-5-((5-(2-methyl-4-(oxetan-3-yl)piperazin-1-yl)pyridin-2-yl)amino)-6-oxo-1,6-dihydro-(3,4'-bipyridin)-2'-yl)-7,7-dimethyl-3,4,7,8-tetrahydro-2h-cyclopenta(4,5)pyrrolo(1,2-a)pyrazin-1(6h)-one
2. Gdc-0853
1. Gdc-0853
2. 1434048-34-6
3. Rg7845
4. Gdc0853
5. Rg-7845
6. Fenebrutinib [inn]
7. Fenebrutinib [usan]
8. Ro7010939
9. E9l2885wul
10. (s)-2-(3'-(hydroxymethyl)-1-methyl-5-((5-(2-methyl-4-(oxetan-3-yl)piperazin-1-yl)pyridin-2-yl)amino)-6-oxo-1,6-dihydro-[3,4'-bipyridin]-2'-yl)-7,7-dimethyl-3,4,7,8-tetrahydro-2h-cyclopenta[4,5]pyrrolo[1,2-a]pyrazin-1(6h)-one
11. Chembl4065122
12. G02599853
13. Ro-7010939
14. G-02599853
15. 10-[3-(hydroxymethyl)-4-[1-methyl-5-[[5-[(2s)-2-methyl-4-(oxetan-3-yl)piperazin-1-yl]pyridin-2-yl]amino]-6-oxopyridin-3-yl]pyridin-2-yl]-4,4-dimethyl-1,10-diazatricyclo[6.4.0.02,6]dodeca-2(6),7-dien-9-one
16. 2-[3'-(hydroxymethyl)-1-methyl-5-({5-[(2s)-2-methyl-4-(oxetan-3-yl)piperazin-1-yl]pyridin-2-yl}amino)-6-oxo[1,6-dihydro[3,4'-bipyridine]]-2'-yl]-7,7-dimethyl-3,4,7,8-tetrahydro-2h-cyclopenta[4,5]pyrrolo[1,2-a]pyrazin-1(6h)-one
17. Fenebrutinib (usan/inn)
18. Fenebrutinib [usan:inn]
19. Unii-e9l2885wul
20. Fenebrutinib (gdc-0853)
21. Fenebrutinib [who-dd]
22. Gtpl9299
23. Schembl14912882
24. Bcp19175
25. Ex-a1308
26. Gdc 0853
27. Gdc-0853gdc-0853
28. Bdbm50244440
29. Nsc798202
30. S8421
31. Zinc220197997
32. Cs-5927
33. Db14785
34. Gdc-0853(rg7845)
35. Nsc-798202
36. Ac-32585
37. Bs-15120
38. Hy-19834
39. Rg-7845 (gdc-0853)
40. Example 130 [us20140194408]
41. J3.615.209e
42. A16339
43. D11457
44. A904370
45. Q23304817
46. (s)-2-(3'-(hydroxymethyl)-1-methyl-5-((5-(2-methyl- 4-(oxetan-3-yl)piperazin-1-yl)pyridin-2-yl)amino)-6-oxo-1,6-dihydro-(3,4'-bipyridin)-2'-yl)-7,7-dimethyl-2,3,4,6,7,8-hexahydro-1h-cyclopenta (4,5)pyrrolo(1,2-a)pyrazin-1-one
47. 2-(3'-(hydroxymethyl)-1-methyl-5-((5-(2-methyl-4-(oxetan-3-yl)piperazin-1-yl)pyridin-2-yl)amino)-6-oxo-1,6-dihydro-(3,4'-bipyridin)-2'-yl)-7,7-dimethyl-3,4,7,8-tetrahydro-2h-cyclopenta(4,5)pyrrolo(1,2-a)pyrazin-1(6h)-one
48. 2h-cyclopenta(4,5)pyrrolo(1,2-a)pyrazin-1(6h)-one, 2-(1,6-dihydro-3'-(hydroxymethyl)-1-methyl-5-((5-((2s)-2-methyl-4-(3-oxetanyl)-1-piperazinyl)-2-pyridinyl)amino)-6-oxo(3,4'-bipyridin)-2'-yl)-3,4,7,8-tetrahydro-7,7-dimethyl-
49. 2h-cyclopenta(4,5)pyrrolo(1,2-a)pyrazin-1(6h)-one, 2-(1,6-dihydro-3'-(hydroxymethyl)-1-methyl-5-((5-((2s)-2-methyl-4-(3-oxetanyl)-1-piperazinyl)-2-pyridinyl)amino)-6-oxo(3,4'-bipyridin)-2'-yl)3,4,7,8-tetrahydro-7,7-dimethyl-
50. 9aj
| Molecular Weight | 664.8 g/mol |
|---|---|
| Molecular Formula | C37H44N8O4 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 7 |
| Exact Mass | 664.34855191 g/mol |
| Monoisotopic Mass | 664.34855191 g/mol |
| Topological Polar Surface Area | 119 Ų |
| Heavy Atom Count | 49 |
| Formal Charge | 0 |
| Complexity | 1340 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Treatment of multiple sclerosis


