
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
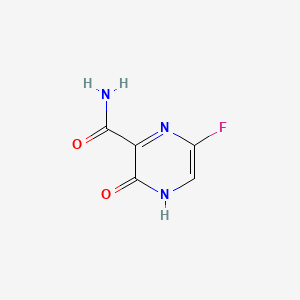

1. 6-fluoro-3-hydroxy-2-pyrazinecarboxamide
2. Avigan
3. Fabiflu
4. T-705 Cpd
1. 259793-96-9
2. 6-fluoro-3-hydroxypyrazine-2-carboxamide
3. 6-fluoro-3-oxo-3,4-dihydropyrazine-2-carboxamide
4. T-705
5. Avigan
6. 5-fluoro-2-oxo-1h-pyrazine-3-carboxamide
7. Favipiravir (t-705)
8. 6-fluoro-3-hydroxy-2-pyrazinecarboxamide
9. T 705
10. Pyrazinecarboxamide, 6-fluoro-3,4-dihydro-3-oxo-
11. 6-fluoro-3-hydroxy-pyrazine-2-carboxamide
12. Favipiravir; T-705
13. Ew5gl2x7e0
14. T705
15. Mfcd12032148
16. 2-pyrazinecarboxamide, 6-fluoro-3-hydroxy-
17. 6-fluoro-3,4-dihydro-3-oxo-2-pyrazinecarboxamide
18. Favipiravir [inn]
19. T-705 Cpd
20. Unii-ew5gl2x7e0
21. Fapilavir
22. Favilavir
23. Favipiravir [usan:inn:jan]
24. Avigan (tn)
25. Favipiravir [mi]
26. Favipiravir [jan]
27. Favipiravir [usan]
28. Favipiravir [who-dd]
29. Schembl587913
30. Favipiravir (jan/usan/inn)
31. Ampz0004
32. Chembl221722
33. Schembl15157866
34. Gtpl11139
35. Dtxsid60948878
36. Chebi:134722
37. Bcpp000056
38. Bcp02422
39. Ex-a2285
40. Bbl104098
41. S7975
42. Stl557913
43. Zinc13915654
44. Akos005166863
45. Akos015995178
46. Zinc584639712
47. Zinc584639713
48. Ccg-266269
49. Cs-0612
50. Db12466
51. Pb25591
52. Pb42412
53. Ncgc00373041-03
54. Ncgc00373041-06
55. Ac-28900
56. Da-19682
57. Hy-14768
58. Ms-20791
59. Sy110833
60. 6-fluoro-3-hydroxypyrazine-2-carbo Xamide
61. Am20080851
62. Ft-0686297
63. Ft-0701282
64. A25520
65. D09537
66. 6-fluoro-3,4-dihydro-3-oxo-pyrazinecarboxamide
67. 793p969
68. A902150
69. J-518718
70. T 705,cas;259793-96-9
71. Q16934561
| Molecular Weight | 157.10 g/mol |
|---|---|
| Molecular Formula | C5H4FN3O2 |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 157.02875454 g/mol |
| Monoisotopic Mass | 157.02875454 g/mol |
| Topological Polar Surface Area | 84.6 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 282 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
In 2014, favipiravir was approved in Japan to treat cases of influenza that were unresponsive to conventional treatment. Given its efficacy at targetting several strains of influenza, it has been investigated in other countries to treat novel viruses including Ebola and most recently, COVID-19.
Favipiravir functions as a prodrug and undergoes ribosylation and phosphorylation intracellularly to become the active favipiravir-RTP. Favipiravir-RTP binds to and inhibits RNA dependent RNA polymerase (RdRp), which ultimately prevents viral transcription and replication.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AX - Other antivirals
J05AX27 - Favipiravir
Absorption
The bioavailability of favipiravir is almost complete at 97.6%. The mean Cmax for the recommended dosing schedule of favipiravir is 51.5 ug/mL. Studies comparing the pharmacokinetic effects of multiple doses of favipiravir in healthy American and Japanese subjects are below: Japanese subjects First Dose: Cmax = 36.24 ug/mL tmax = 0.5 hr AUC = 91.40 ugxhr/mL American subjects First Dose: Cmax = 22.01 ug/mL tmax = 0.5 hr AUC = 44.11 ugxhr/mL Japanese Subjects Final Dose: Cmax = 36.23 ug/mL Tmax = 0.5 hr AUC = 215.05 ugxhr/mL American Subjects Final Dose: Cmax = 23.94 ug/mL Tmax = 0.6 hr AUC = 73.27 ugxhr/mL When favipiravir was given as a single dose of 400 mg with food, the Cmax decreased. It appears that when favipiravir is given at a higher dose or in multiple doses, irreversible inhibition of aldehyde oxidase (AO) occurs and the effect of food on the Cmax is lessened.
Route of Elimination
Favipiravir's metabolites are predominantly renally cleared.
Volume of Distribution
The apparent volume of distribution of favipiravir is 15 - 20 L.
Clearance
The recommended oral dosing regimen for favipiravir is as follows: Day 1: 1600 mg twice daily; Days 2-5: 600 mg twice daily. The reported CL/F for favipiravir 1600 mg dosed once daily is 2.98 L/hr 0.30 and the CL/F values for favipiravir 600 mg dosed twice daily on days 1-2 and once daily on days 3-7 were 6.72 L/hr 1.68 on Day 1, and 2.89 L/hr 0.91 on Day 7. There is currently no reported clearance data for favipiravir 1600 mg dosed twice daily.
Favipiravir is extensively metabolized with metabolites excreted mainly in the urine. The antiviral undergoes hydroxylation primarily by aldehyde oxidase and to a lesser extent by xanthine oxidase to the inactive metabolite, T705M1.
The elimination half-life of favipiravir is estimated to range from 2 to 5.5 hours.
The mechanism of action of favipiravir is novel compared to existing influenza antivirals that primarily prevent entry and exit of the virus from cells. The active favipiravir-RTP selectively inhibits RNA polymerase and prevents replication of the viral genome. There are several hypotheses as to how favipiravir-RTP interacts with RNA dependent RNA polymerase (RdRp). Some studies have shown that when favipiravir-RTP is incorporated into a nascent RNA strand, it prevents RNA strand elongation and viral proliferation. Studies have also found that the presence of purine analogs can reduce favipiravirs antiviral activity, suggesting competition between favipiravir-RTP and purine nucleosides for RdRp binding. Although favipiravir was originally developed to treat influenza, the RdRp catalytic domain (favipiravir's primary target), is expected to be similar for other RNA viruses. This conserved RdRp catalytic domain contributes to favipiravir's broad-spectrum coverage.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Market Place

ABOUT THIS PAGE
28
PharmaCompass offers a list of Favipiravir API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Favipiravir manufacturer or Favipiravir supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Favipiravir manufacturer or Favipiravir supplier.
PharmaCompass also assists you with knowing the Favipiravir API Price utilized in the formulation of products. Favipiravir API Price is not always fixed or binding as the Favipiravir Price is obtained through a variety of data sources. The Favipiravir Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Favipiravir manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Favipiravir, including repackagers and relabelers. The FDA regulates Favipiravir manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Favipiravir API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Favipiravir manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Favipiravir supplier is an individual or a company that provides Favipiravir active pharmaceutical ingredient (API) or Favipiravir finished formulations upon request. The Favipiravir suppliers may include Favipiravir API manufacturers, exporters, distributors and traders.
click here to find a list of Favipiravir suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Favipiravir DMF (Drug Master File) is a document detailing the whole manufacturing process of Favipiravir active pharmaceutical ingredient (API) in detail. Different forms of Favipiravir DMFs exist exist since differing nations have different regulations, such as Favipiravir USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Favipiravir DMF submitted to regulatory agencies in the US is known as a USDMF. Favipiravir USDMF includes data on Favipiravir's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Favipiravir USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Favipiravir suppliers with USDMF on PharmaCompass.
A Favipiravir written confirmation (Favipiravir WC) is an official document issued by a regulatory agency to a Favipiravir manufacturer, verifying that the manufacturing facility of a Favipiravir active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Favipiravir APIs or Favipiravir finished pharmaceutical products to another nation, regulatory agencies frequently require a Favipiravir WC (written confirmation) as part of the regulatory process.
click here to find a list of Favipiravir suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Favipiravir as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Favipiravir API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Favipiravir as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Favipiravir and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Favipiravir NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Favipiravir suppliers with NDC on PharmaCompass.
Favipiravir Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Favipiravir GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Favipiravir GMP manufacturer or Favipiravir GMP API supplier for your needs.
A Favipiravir CoA (Certificate of Analysis) is a formal document that attests to Favipiravir's compliance with Favipiravir specifications and serves as a tool for batch-level quality control.
Favipiravir CoA mostly includes findings from lab analyses of a specific batch. For each Favipiravir CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Favipiravir may be tested according to a variety of international standards, such as European Pharmacopoeia (Favipiravir EP), Favipiravir JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Favipiravir USP).

