



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
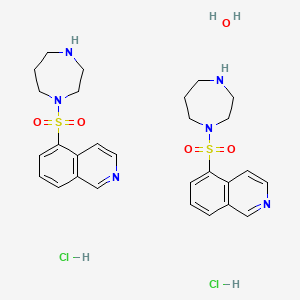

1. 186694-02-0
2. Fasudil Hydrochloride Hemihydrate
3. Eril-s
4. Fasudil Hcl Semihydrate
5. Fasudil Hydrochloride Hydrate [jan]
6. Li4l0r5y7t
7. 5-(hexahydro-1h-1,4-diazepine-1-ylsulfonyl)isoquinoline Hydrochloride Hemihydrate
8. 5-(1,4-diazepan-1-ylsulfonyl)isoquinoline;hydrate;dihydrochloride
9. 5-((1,4-diazepan-1-yl)sulfonyl)isoquinoline Hydrochloride Hemihydrate
10. 1h-1,4-diazepine, Hexahydro-1-(5-isoquinolinylsulfonyl)-, Monohydrochloride, Hydrate (2:1)
11. Isoquinoline, 5-((hexahydro-1h-1,4-diazepin-1-yl)sulfonyl)-, Hydrochloride, Hydrate (2:2:1)
12. Fasudil Hydrochloride Hydrate (jan)
13. 5-(1,4-diazepan-1-ylsulfonyl)isoquinoline,hydrate,dihydrochloride;5-(1,4-diazepan-1-ylsulfonyl)isoquinoline,hydrate,dihydrochloride
14. Hexahydro-1-(5-isoquinolinesulfonyl)-1h-1,4-diazepine Monohydrochloride Hemihydrate;hexahydro-1-(5-isoquinolinesulfonyl)-1h-1,4-diazepine Monohydrochloride Hemihydrate
15. Unii-li4l0r5y7t
16. Fasudil Hcl Hemihydrate
17. Eril-s (tn)
18. Dtxsid80171929
19. Chebi:180695
20. Fasudil (hydrochloride Semihydrate)
21. Bcp30939
22. D03115
23. F74621
24. A899480
25. Q27282992
26. 5-((1,4-diazepan-1-yl)sulfonyl)isoquinolinehydrochloridehemihydrate
27. 5-(1,4-diazepan-1-ylsulfonyl)isoquinoline Hydrochloride Hydrate (2:2:1)
28. 5-(1,4-diazepane-1-sulfonyl)isoquinoline--hydrogen Chloride--water (2/2/1)
29. Fasudil Hydrochloride Hemihydrate;fasudil Hcl Semihydrate;ha-1077; Ha1077; Ha 1077
| Molecular Weight | 673.7 g/mol |
|---|---|
| Molecular Formula | C28H38Cl2N6O5S2 |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 672.1722161 g/mol |
| Monoisotopic Mass | 672.1722161 g/mol |
| Topological Polar Surface Area | 142 Ų |
| Heavy Atom Count | 43 |
| Formal Charge | 0 |
| Complexity | 421 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 5 |


