



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. A0026
2. Bay 56-6854
3. Bay 566854
4. Bay-56-6854
5. Bay-566854
6. Bay56-6854
7. Bay566854
8. Faropenem Medoxomil
9. Sun 208
10. Sun A0026
11. Sun-208
12. Sun-a0026
13. Sun208
1. 141702-36-5
2. Faropenem Medoxomil
3. Faropenem Medoxil
4. Sun-a0026
5. Fropenem Daloxate
6. Faropenem Medoxomil [usan]
7. Bay-56-6854
8. A-0026
9. 5ok523o4fu
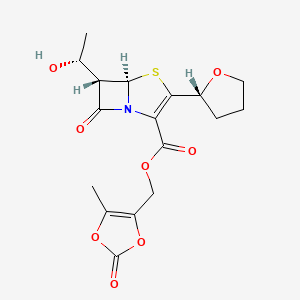
10. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl (5r,6s)-6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-oxolan-2-yl]-4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylate
11. A0026
12. Sun-208
13. Faropenem Medoxomil (usan)
14. Orapem
15. 4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid, 6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-tetrahydro-2-furanyl]-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester, (5r,6s)-
16. Unii-5ok523o4fu
17. Faropenem-medoxomil
18. Bay 566854
19. Bay 56-6854
20. A 0026
21. Schembl2334640
22. Sun208
23. Faropenem Daloxate [mi]
24. Chembl1257070
25. Sun A0026
26. Sun 208
27. Chebi:134710
28. Zinc3806644
29. Bay566854
30. Bay-566854
31. Bay56-6854
32. Cs-0390
33. Db05659
34. Hy-10004
35. D08919
36. 702f365
37. A911194
38. Q27095691
39. (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl (5r,6s)-6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydrofuran-2-yl)-4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylate
40. (5-methyl-2-oxo-1,3-dioxoren-4-yl)methyl(5r,6s)-6-((r)-1-hydroxyethyl)-7-oxo-3-((r)-2-tetrahydrofuryl)-4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylate
41. (5r,6s)-6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-oxolane-2-yl]-4-thia-1-azabicyclo[3.2.0]hepta-2-ene-2-carboxylic Acid 5-methyl-2-oxo-1,3-dioxole-4-ylmethyl Ester
42. 4-thia-1-azabicyclo(.32.0)hept-2-ene-2-carboxylic Acid, 6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydro-2-furanyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester, (5r,6s)-
43. 4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-((1r)-1-hydroxyethyl)-7-oxo-3-((2r)-tetrahydro-2-furanyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester, (5r,6s)
44. 4-thia-1-azabicyclo(3.2.0)hept-2-ene-2-carboxylic Acid, 6-(1-hydroxyethyl)-7-oxo-3-(tetrahydro-2-furanyl)-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester, (5r-(3(r*),5alpha,6alpha(r*)))-
45. 4-thia-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic Acid, 6-[(1r)-1-hydroxyethyl]-7-oxo-3-[(2r)-tetrahydro-2-furanyl]-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl Ester
| Molecular Weight | 397.4 g/mol |
|---|---|
| Molecular Formula | C17H19NO8S |
| XLogP3 | 0.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 397.08313774 g/mol |
| Monoisotopic Mass | 397.08313774 g/mol |
| Topological Polar Surface Area | 137 Ų |
| Heavy Atom Count | 27 |
| Formal Charge | 0 |
| Complexity | 775 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in bacterial infection, bronchitis, otitis media, and pediatric indications.
Faropenem has demonstrated excellent in vitro activity against common respiratory pathogens, many aerobic gram-positive organisms, and anaerobes. Activity against gram-negative organisms is more reserved. In vivo data suggest that faropenem is efficacious in treating community-acquired infections including uncomplicated skin and skin structure infections; however, more data may help to characterize faropenem's place in antimicrobial therapy.
Like other beta-lactam antibiotics, faropenem acts by inhibiting the synthesis of bacterial cell walls. It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the cell wall of Gram-positive bacteria. It does this by binding to and competitively inhibiting the transpeptidase enzyme used by bacteria to cross-link the peptide (D-alanyl-alanine) used in peptidogylcan synthesis.


