



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
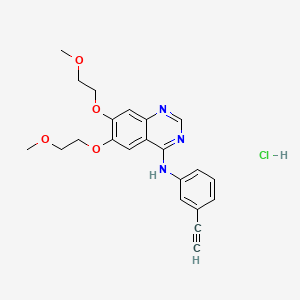

1. 11c Erlotinib
2. 11c-erlotinib
3. 358,774, Cp
4. 358774, Cp
5. Cp 358,774
6. Cp 358774
7. Cp-358,774
8. Cp-358774
9. Cp358,774
10. Cp358774
11. Erlotinib
12. Erlotinib Hcl
13. Hcl, Erlotinib
14. Hydrochloride, Erlotinib
15. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine
16. Osi 774
17. Osi-774
18. Osi774
19. Tarceva
1. 183319-69-9
2. Erlotinib Hcl
3. Tarceva
4. Osi-774
5. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine Hydrochloride
6. Osi 774
7. Erlotinib (hydrochloride)
8. Cp-358774
9. Cp 358774
10. Erlotinib, Hydrochloride Salt
11. Tarceva (erlotinib Hydrochloride)
12. Nsc 718781
13. Cp-358,774-01
14. Da87705x9k
15. 6,7-bis(2-methoxyethoxy)-4-(3-ethynylanilino)quinazoline Hydrochloride
16. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine;hydrochloride
17. Cp-358
18. 4-quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-, Hydrochloride (1:1)
19. Nsc-718781
20. 6,7-bis(2-methoxyethoxy)-4-(3-ethynylanilino)quinazoline Hcl
21. 183319-69-9 (hcl)
22. 4-(m-ethynylanilino)-6,7-bis(2-methoxyethoxy)quinazoline Monohydrochloride
23. Cp-358774-01
24. 4-quinazolinamine, N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-, Monohydrochloride
25. [6,7-bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine Hydrochloride
26. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine Monohydrochloride.
27. Chebi:53509
28. Osi-744
29. Smr002529980
30. Erlotinib Hydrochloride [usan]
31. Nsc718781
32. 183319-69-9 Pound Not183321-74-6
33. Erlotinib Hcl (osi-744)
34. Unii-da87705x9k
35. Erlotinib, Hcl
36. Erlotinib Hcl Salt
37. Erlotinib Hydrochloride [usan:inn]
38. Tarceva (osi)
39. Tarceva Hydrochloride
40. Mfcd07781272
41. Erlotonib Hydrochloride
42. Erlotinib Hydrochlroide
43. Erlotinib(osi-744)
44. Mls003899192
45. Mls004774139
46. Chembl1079742
47. Nsc 718781) Hcl
48. Dtxsid10171412
49. Ex-a064
50. Erlotinib Hydrochloride (tarceva)
51. Bcpp000238
52. Bcp02600
53. Erlotinib Hydrochloride [mi]
54. Ac-400
55. Erlotinib Hydrochloride [jan]
56. S1023
57. Akos015849087
58. Bcp9000658
59. Ccg-269002
60. Cs-0123
61. Erlotinib Hydrochloride [mart.]
62. Ks-1202
63. Pb30965
64. Sb16917
65. Erlotinib Hydrochloride [usp-rs]
66. Erlotinib Hydrochloride [who-dd]
67. Ro-50-8231
68. Be164421
69. Bp-30224
70. Hy-12008
71. (cp358774
72. Db-011534
73. Am20090622
74. Erlotinib Hydrochloride [orange Book]
75. Ft-0651479
76. Ec-000.2313
77. E-4007
78. 319e699
79. Q27124083
80. F0001-2385
81. Erlotinib Hydrochloride Is Known As A Egfr Kinase Inhibitor.
82. Erlotinib Hydrochloride,cp-358774, Osi-774, Nsc 718781
83. 6,7-bis-(2-methoxyethoxy)-4-(3-ethynylanilino)quinazoline Hydrochloride
84. [6,7-bis(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl- Phenyl)amine Hydrochloride
85. N-(3-ethenylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine Hydrochloride
86. N-(3-ethynylphenyl)-6,7-bis(1-methoxyethoxy)-4-quinazolinamine Hydrochloride
87. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine, Monohydrochloride
88. N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-quinazolin-4-amine Hydrochloride
| Molecular Weight | 429.9 g/mol |
|---|---|
| Molecular Formula | C22H24ClN3O4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 11 |
| Exact Mass | 429.1455339 g/mol |
| Monoisotopic Mass | 429.1455339 g/mol |
| Topological Polar Surface Area | 74.7 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 525 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Erlotinib hydrochloride |
| Drug Label | TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:Erloti... |
| Active Ingredient | Erlotinib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 150mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Mylan Pharms |
| 2 of 4 | |
|---|---|
| Drug Name | Tarceva |
| PubMed Health | Erlotinib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Drug Label | TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:Erloti... |
| Active Ingredient | Erlotinib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 150mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Osi Pharms |
| 3 of 4 | |
|---|---|
| Drug Name | Erlotinib hydrochloride |
| Drug Label | TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:Erloti... |
| Active Ingredient | Erlotinib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 150mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Mylan Pharms |
| 4 of 4 | |
|---|---|
| Drug Name | Tarceva |
| PubMed Health | Erlotinib (By mouth) |
| Drug Classes | Antineoplastic Agent, Immunological Agent |
| Drug Label | TARCEVA (erlotinib), a kinase inhibitor, is a quinazolinamine with the chemical name N-(3-ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine. TARCEVA contains erlotinib as the hydrochloride salt that has the following structural formula:Erloti... |
| Active Ingredient | Erlotinib hydrochloride |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 100mg base; eq 150mg base; eq 25mg base |
| Market Status | Prescription |
| Company | Osi Pharms |
* Non-small cell lung cancer (NSCLC):
Tarceva is also indicated for switch maintenance treatment in patients with locally advanced or metastatic non-small cell lung cancer with EGFR activating mutations and stable disease after first-line chemotherapy.
Tarceva is also indicated for the treatment of patients with locally advanced or metastatic non-small cell lung cancer after failure of at least one prior chemotherapy regimen.
In patients with tumours without EGFR activating mutations, Tarceva is indicated when other treatment options are not considered suitable.
When prescribing Tarceva, factors associated with prolonged survival should be taken into account.
No survival benefit or other clinically relevant effects of the treatment have been demonstrated in patients with Epidermal Growth Factor Receptor (EGFR)-IHC - negative tumours.
* Pancreatic cancer :
Tarceva in combination with gemcitabine is indicated for the treatment of patients with metastatic pancreatic cancer .
When prescribing Tarceva, factors associated with prolonged survival should be taken into account.
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EB02








