



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
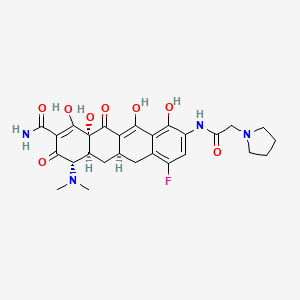

1. (4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-((pyrrolidin-1-ylacetyl)amino)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
2. (4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-((pyrrolidin-1-ylacetyl)amino)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide Dihydrochloride
3. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline
4. Eravacycline Dihydrochloride
5. Tp-434
6. Tp-434-046
7. Tp-434046
8. Tp434
9. Xerava
1. 1207283-85-9
2. Tp-434
3. Xerava
4. Eravacycline [usan]
5. Tp434
6. (4s,4as,5ar,12ar)-4-(dimethylamino)-7-fluoro-1,10,11,12a-tetrahydroxy-3,12-dioxo-9-[(2-pyrrolidin-1-ylacetyl)amino]-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
7. Eravacycline (usan)
8. 07896928zc
9. 1-pyrrolidineacetamide, N-((5ar,6as,7s,10as)-9-(aminocarbonyl)-7-(dimethylamino)-4-fluoro-5,5a,6,6a,7,10,10a,12-octahydro-1,8,10a,11-tetrahydroxy-10,12-dioxo-2-naphthacenyl)-
10. Eravacycline [usan:inn]
11. Unii-07896928zc
12. Yqm
13. Tp434;eravacycline
14. Eravacycline [mi]
15. Eravacycline [inn]
16. Eravacycline [who-dd]
17. Chembl1951095
18. Chembl4597183
19. Schembl10040430
20. Gtpl10805
21. Ex-a751
22. Chebi:177804
23. Dtxsid401026285
24. Mfcd31010183
25. Zinc200151468
26. Cs-7564
27. Db12329
28. Compound 17j [pmid: 2148514]
29. Hy-16980
30. D10369
31. J-690064
32. Q15410941
33. 7-fluoro-9-pyrrolidinoacetamido-6-demethyl-6-deoxytetracycline
34. (4s,4as,5ar,12ar)-4-(dimethylamino)-7-fluoro-1,10,11,12a-tetrahydroxy-3,12-dioxo-9-[2-(pyrrolidin-1-yl)acetamido]-3,4,4a,5,5a,6,12,12a-octahydrotetracene-2-carboxamide
35. (4s,4as,5ar,12ar)-4-(dimethylamino)-7-luoro-1,10,11,12a-tetrahydroxy-3,12-dioxo-9-[(2-pyrrolidin-1-ylacetyl)amino]-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
36. (4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-((pyrrolidin-1-ylacetyl)amino)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
37. (4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-[(2-pyrrolidin-1-ylacetyl)amino]-4a,5,5a,6-tetrahydro-4h-tetracene-2-carboxamide
38. (4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12atetrahydroxy-1,11-dioxo-9-(2-(pyrrolidin-1-yl)acetamido)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
39. 4s,4as,5ar,12as)-4-(dimethylamino)-7-fluoro-3,10,12,12a-tetrahydroxy-1,11-dioxo-9-((pyrrolidin-1-ylacetyl)amino)-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide
40. 7-[6-(2-hydroxy-2-propanyl)-3-pyridinyl]-1-(4-methoxycyclohexyl)-3,4-dihydropyrazino[2,3-b]pyrazin-2(1h)-one
41. N-[(5ar,6as,7s,10as)-9-(aminocarbonyl)-7-(dimethylamino)-4-fluoro-5,5a,6,6a,7,10,10a,12-octahydro-1,8,10a,11-tetrahydroxy-10,12-dioxo-2-naphthacenyl]-1-pyrrolidineacetamide
| Molecular Weight | 558.6 g/mol |
|---|---|
| Molecular Formula | C27H31FN4O8 |
| XLogP3 | 1 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 5 |
| Exact Mass | 558.21259212 g/mol |
| Monoisotopic Mass | 558.21259212 g/mol |
| Topological Polar Surface Area | 194 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 1200 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Eravacycline is a tetracycline class antibacterial indicated for the treatment of complicated intra-abdominal infections in patients 18 years of age and older.
FDA Label
Xerava is indicated for the treatment of complicated intra-abdominal infections (cIAI) in adults.
Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Eravacycline is an antibiotic that disrupts bacterial protein synthesis, treating complicated intraabdominal infections.
J01AA
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01A - Tetracyclines
J01AA - Tetracyclines
J01AA13 - Eravacycline
Absorption
Following single-dose intravenous administration, eravacycline AUC (area under the curve) and Cmax (maximum concentration) increase dose-proportionally for doses from 1 mg/kg - 3 mg/kg (3 times the approved dose). There is approximately 45% accumulation following intravenous dosing of 1 mg/kg every 12 hours.
Route of Elimination
Following a single intravenous dose of radiolabeled eravacycline 60 mg, approximately 34% of the dose is excreted in urine and 47% in feces as unchanged eravacycline (20% in urine and 17% in feces) and metabolites.
Volume of Distribution
The volume of distribution at steady-state is approximately 321 L.
Clearance
17.82 L/min (standard deviation of 3.4).
Eravacycline is metabolized primarily by CYP3A4- and FMO-mediated oxidation.
The mean elimination half-life is 20 hours.
Eravacycline is a fluorocycline antibacterial of the tetracycline class of antibacterial drugs. Eravacycline disrupts bacterial protein synthesis by binding to the 30S ribosomal subunit, preventing the incorporation of amino acid residues into elongating peptide chains. In general, eravacycline is bacteriostatic against gram-positive bacteria (e.g., Staphylococcus aureus and Enterococcus faecalis); however, in vitro bactericidal activity has been shown against certain strains of Escherichia coli and Klebsiella pneumoniae.


