



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe

Canada
0

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
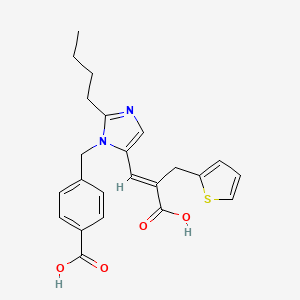

1. 2-thiophenepropanoic Acid, Alpha-((2-butyl-1-((4- Carboxyphenyl)methyl)-lh-imidazol-5-yl)methylene)-, (e)-
2. Sk And F 108566
3. Skf-108566
4. Teveten
1. 133040-01-4
2. Teveten
3. Sk&f 108566
4. Sk&f-108566
5. (e)-2-butyl-1-(p-carboxybenzyl)-alpha-2-thenylimidazole-5-acrylic Acid
6. (e)-4-((2-butyl-5-(2-carboxy-3-(thiophen-2-yl)prop-1-en-1-yl)-1h-imidazol-1-yl)methyl)benzoic Acid
7. Chembl813
8. Sk-108566
9. 2kh13z0s0y
10. Chebi:4814
11. (e)-3-[2-n-butyl-1-{(4-carboxyphenyl)methyl}-1h-imidazol-5-yl]-2-(2-thienyl)methyl-2-propenoic Acid
12. (e)-alpha{[2-butyl-1-[(4-carboxyphenyl)methyl]-1h-imidazole-5-yl]methylene}-2-thiopheneproprionic Acid
13. 4-({2-butyl-5-[(1e)-2-carboxy-3-(2-thienyl)prop-1-en-1-yl]-1h-imidazol-1-yl}methyl)benzoic Acid
14. 4-[[2-butyl-5-[(e)-2-carboxy-3-thiophen-2-ylprop-1-enyl]imidazol-1-yl]methyl]benzoic Acid
15. 4-[[2-butyl-5-[(e)-3-hydroxy-3-oxo-2-(thiophen-2-ylmethyl)prop-1-enyl]imidazol-1-yl]methyl]benzoic Acid
16. [3h]eprosartan
17. [3h]-eprosartan
18. 4-({2-butyl-5-[(1e)-2-carboxy-2-(thiophen-2-ylmethyl)eth-1-en-1-yl]-1h-imidazol-1-yl}methyl)benzoic Acid
19. Ncgc00164557-01
20. Eprosartan (usan/inn)
21. Unii-2kh13z0s0y
22. Eprozar
23. Eprosartan [usan:inn:ban]
24. Sr-05000001450
25. Hsdb 7521
26. [3h]sk&f 108566
27. Eprosartan [mi]
28. Eprosartan [inn]
29. Eprosartan [hsdb]
30. Eprosartan [usan]
31. Eprosartan [vandf]
32. Schembl4025
33. Eprosartan [who-dd]
34. Gtpl588
35. 4-({2-butyl-5-[(1e)-2-carboxy-3-(thiophen-2-yl)prop-1-en-1-yl]-1h-imidazol-1-yl}methyl)benzoic Acid
36. Bidd:gt0030
37. Gtpl3940
38. Dtxsid0022989
39. Chebi:94094
40. 4-[[2-butyl-5-(2-carboxy-3-thiophen-2-ylprop-1-enyl)-1-imidazolyl]methyl]benzoic Acid
41. Bcpp000239
42. Hms2089o10
43. Bcp02353
44. Bdbm50011977
45. Stk618317
46. Zinc29319828
47. Akos005552473
48. Bcp9000656
49. Db00876
50. Skb 108566
51. Ncgc00164557-06
52. Ncgc00164557-11
53. Ncgc00164557-13
54. (e)-4-((2-butyl-5-(2-carboxy-3-(thiophen-2-yl)prop-1-enyl)-1h-imidazol-1-yl)methyl)benzoic Acid
55. 2-thiophenepropanoic Acid, Alpha-((2-butyl-1-((4-carboxyphenyl)methyl)-lh-imidazol-5-yl)methylene)-, (e)-
56. Bs-20679
57. Hy-117743
58. Cs-0067582
59. 40e014
60. C07467
61. D04040
62. Ab01275448-01
63. Ab01275448_02
64. L000248
65. Q784717
66. J-006289
67. Sr-05000001450-1
68. Brd-k67977190-066-01-5
69. (e)-2-butyl-1-(p-carboxybenzyl)-.alpha.-2-thenylimidazole-5-acrylic Acid
70. 1-[4-carboxybenzyl]-2-butyl-alpha-[(2-thienyl)methyl]-1h-imidazole-5-propenoic Acid
71. 4-[[2-butyl-5-(2-carboxy-3-thiophen-2-yl-prop-1-enyl)imidazol-1-yl]methyl]benzoic Acid
72. (alphae)-alpha-[[2-butyl-1-[(4-carboxyphenyl)methyl]-1h-imidazol-5-yl]methylene]-2-thiophenepropanoic Acid
73. (e)-3-[2-butyl-1-[(4-carboxyphenyl)methyl]imidazol-5-yl]-2-(2-thienylmethyl)-2-propenoic Acid
74. (e)-alpha-[[2-butyl-1-[(4-carboxyphenyl)-methyl]-1h-imidazol-5-yl]methylene]-2-thiophene Propanoic Acid
75. (e)-alpha-[[2-butyl-1-[(4carboxyphenyl)methyl]-1h-imidazol-5-yl]methylene]-2-thiophene Propanoic Acid
76. 2-thiophenepropanoic Acid, .alpha.-((2-butyl-1-((4-carboxyphenyl)methyl)-lh-imidazol-5-yl)methylene)-, (e)-
| Molecular Weight | 424.5 g/mol |
|---|---|
| Molecular Formula | C23H24N2O4S |
| XLogP3 | 4.5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 10 |
| Exact Mass | 424.14567842 g/mol |
| Monoisotopic Mass | 424.14567842 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 618 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Angiotensin II Type 2 Receptor Blockers; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Eprosartan. Online file (MeSH, 2014). Available from, as of September 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Teveten is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensives such as diuretics and calcium channel blockers. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Both angiotensin II receptor antagonists /including eprosartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2063
Angiotensin II receptor antagonists /inlcuding eprosartan/ have been used in the management of congestive heart failure. While angiotensin II receptor antagonists appear to share the hemodynamic effects of ACE inhibitors, some experts state that, in the absence of data documenting comparable long-term cardiovascular and/or renal benefits, angiotensin II receptor antagonists should be reserved principally for patients in whom ACE inhibitors are indicated but who are unable to tolerate the drugs (e.g., because of intractable cough or angioedema). /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2063
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Teveten as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Teveten as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the renin-angiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examination to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Teveten, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Neonates with a history of in utero exposure to Teveten: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
For more Drug Warnings (Complete) data for EPROSARTAN (17 total), please visit the HSDB record page.
For the management of hypertension alone or in combination with other classes of antihypertensive agents. Also used as a first-line agent in the treatment of diabetic nephropathy, as well as a second-line agent in the treatment of congestive heart failure (only in those intolerant of ACE inhibitors).
FDA Label
Angiotensin II, the principal pressor agent of the renin-angiotensin system, is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme [kininase II]. It is responsible for effects such as vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Eprosartan selectively blocks the binding of angiotensin II to the AT1 receptor, which in turn leads to multiple effects including vasodilation, a reduction in the secretion of vasopressin, and reduction in the production and secretion of aldosterone. The resulting effect is a decrease in blood pressure.
Angiotensin II Type 2 Receptor Blockers
Agents that antagonize the ANGIOTENSIN II TYPE 2 RECEPTOR. (See all compounds classified as Angiotensin II Type 2 Receptor Blockers.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C09CA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA02 - Eprosartan
Absorption
Absolute bioavailability following a single 300 mg oral dose of eprosartan is approximately 13%. Administering eprosartan with food delays absorption.
Eprosartan is excreted in animal milk; it is not known whether eprosartan is excreted in human milk.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Plasma protein binding of eprosartan is high (approximately 98%) and constant over the concentration range achieved with therapeutic doses. The pooled population pharmacokinetic analysis from two Phase 3 trials of 299 men and 172 women with mild to moderate hypertension (aged 20 to 93 years) showed that eprosartan exhibited a population mean oral clearance (CL/F) for an average 60-year-old patient of 48.5 L/hr. The population mean steady-state volume of distribution (Vss/F) was 308 L. Eprosartan pharmacokinetics were not influenced by weight, race, gender or severity of hypertension at baseline. Oral clearance was shown to be a linear function of age with CL/F decreasing 0.62 L/hr for every year increase.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Eprosartan is eliminated by biliary and renal excretion, primarily as unchanged compound. Less than 2% of an oral dose is excreted in the urine as a glucuronide. There are no active metabolites following oral and intravenous dosing with (14)C eprosartan in human subjects. Eprosartan was the only drug-related compound found in the plasma and feces. Following intravenous (14)C eprosartan, about 61% of the material is recovered in the feces and about 37% in the urine. Following an oral dose of (14)C eprosartan, about 90% is recovered in the feces and about 7% in the urine.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Absolute bioavailability following a single 300 mg oral dose of eprosartan is approximately 13%. Eprosartan plasma concentrations peak at 1 to 2 hours after an oral dose in the fasted state. Administering eprosartan with food delays absorption, and causes variable changes (<25%) in Cmax and AUC values which do not appear clinically important. Plasma concentrations of eprosartan increase in a slightly less than dose-proportional manner over the 100 mg to 800 mg dose range. The mean terminal elimination half-life of eprosartan following multiple oral doses of 600 mg was approximately 20 hours. Eprosartan does not significantly accumulate with chronic use.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
For more Absorption, Distribution and Excretion (Complete) data for EPROSARTAN (6 total), please visit the HSDB record page.
Eprosartan is not metabolized by the cytochrome P450 system. It is mainly eliminated as unchanged drug. Less than 2% of an oral dose is excreted in the urine as a glucuronide.
Following an oral dose of (14)C eprosartan, about 90% is recovered in the feces and about 7% in the urine. Approximately 20% of the radioactivity excreted in the urine was an acyl glucuronide of eprosartan with the remaining 80% being unchanged eprosartan.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
The terminal elimination half-life of eprosartan following oral administration is typically 5 to 9 hours.
... The mean terminal elimination half-life of eprosartan following multiple oral doses of 600 mg was approximately 20 hours. ...
Physicians Desk Reference 61st ed, Thomson PDR, Montvale, NJ 2007., p. 1735
After oral administration of eprosartan to healthy volunteers ... the drug's terminal elimination half-life is typically 5-9 hours after oral administration. ...
PMID:10213525 Bottorff MB et al; Pharmacotherapy 19 (4 Pt 2): 73S-78S (1999)
Eprosartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues (e.g., vascular smooth muscle, adrenal gland). There is also an AT2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis. Eprosartan does not exhibit any partial agonist activity at the AT1 receptor. Its affinity for the AT1 receptor is 1,000 times greater than for the AT2 receptor. In vitro binding studies indicate that eprosartan is a reversible, competitive inhibitor of the AT1 receptor. Eprosartan has also been shown to bind to AT1 receptors both presynaptically and synaptically. Its action on presynaptic AT1 receptors results in the inhibition of sympathetically stimulated noradrenaline release. Unlike ACE inhibitors, eprosartan and other ARBs do not interfere with response to bradykinins and substance P, which allows for the absence of adverse effects that are present in ACE inhibitors (eg. dry cough).
Angiotensin II (AII) receptor blockers offer an alternative means of blocking the renin-angiotensin-aldosterone system (RAAS) to angiotensin converting enzyme (ACE) inhibitors. Being highly selective for the AII receptor subtype AT(1), AII receptor blockers may avoid side-effects associated with ACE inhibitor treatment, such as cough. Eprosartan is a non-biphenyl, non-tetrazole competitive blocker that is chemically distinct from other AII receptor blockers, which may account for differences in its pharmacological properties. It induces dual blockade of AT(1) receptors both presynaptically and postsynaptically, reducing sympathetic nerve activity to a significantly greater degree than other AT(1) receptor blockers. ...
PMID:12766389 Puig JG et al; Cardiovasc Drugs Ther 16 (6): 543-9 (2002)
Angiotensin II (formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (kininase II)), a potent vasoconstrictor, is the principal pressor agent of the renin-angiotensin system. Angiotensin II also stimulates aldosterone synthesis and secretion by the adrenal cortex, cardiac contraction, renal resorption of sodium, activity of the sympathetic nervous system, and smooth muscle cell growth. Eprosartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor found in many tissues (e.g., vascular smooth muscle, adrenal gland). There is also an AT2 receptor found in many tissues but it is not known to be associated with cardiovascular homeostasis. Eprosartan does not exhibit any partial agonist activity at the AT1 receptor. Its affinity for the AT1 receptor is 1,000 times greater than for the AT2 receptor. In vitro binding studies indicate that eprosartan is a reversible, competitive inhibitor of the AT1 receptor. Blockade of the AT1 receptor removes the negative feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and circulating angiotensin II do not overcome the effect of eprosartan on blood pressure. Teveten does not inhibit kininase II, the enzyme that converts angiotensin I to angiotensin II and degrades bradykinin; whether this has clinical relevance is not known. It does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation.
NIH; DailyMed. Current Medication Information for Teveten (Eprosartan Mesylate) Tablet (Revised: August 2014). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=7cde57a7-eded-4c22-b1c7-98afc5546997
Selective blockade of the angiotensin II AT1 receptor represents a novel mechanism for interrupting the renin-angiotensin system without altering the potential benefits of AT2 receptor stimulation. This selective inhibition produces none of the disadvantages associated with reduced bradykinin metabolism and angiotensin II generated by non-angiotensin-converting enzyme pathways. Eprosartan is a potent (1.4 nmol/L) AT1 receptor antagonist that competitively blocks angiotensin II-induced vascular contraction. In various animal models of disease, including hypertension and stroke, eprosartan is effective in reducing disease progression. Eprosartan also has sympathoinhibitory activity, as demonstrated by an inhibition of the pressor responses induced by activation of sympathetic outflow through spinal cord stimulation in pithed rats. In contrast, some of the other angiotensin II receptor antagonists, such as losartan, at equivalent angiotensin II blocking doses, have no effect on sympathetic nervous system activity. Because eprosartan can inhibit both the direct effects of angiotensin II as well as the indirect effects that are mediated by enhanced sympathetic neurotransmission, this may represent an important advance in the treatment of elevated systolic blood pressure.
PMID:10467220 Brooks DP et al; Am Heart J 138 (3 Pt 2): 246-51 (1999)
Although these agents /angiotensin II receptor antagonists/ are similar to ACE inhibitors in that they decrease the effects of angiotensin II, rather than decreasing the formation of angiotensin II, drugs antagonize angiotensin II at the type I angiotensin receptor. This allows the drugs to inhibit the vasoconstrictive and aldosterone promoting effects of angiotensin II without interfering with bradykinin degradation, significantly reducing the adverse effects of cough and angioedema seen with ACE inhibitor therapy. ... /Angiotensin II receptor antagonists/
Goldfrank, L.R. (ed). Goldfrank's Toxicologic Emergencies. 7th Edition McGraw-Hill New York, New York 2002., p. 782


