



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
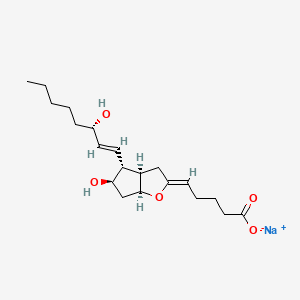

1. Epoprostanol
2. Epoprostenol
3. Epoprostenol Sodium Salt, (5z,9alpha,11alpha,13e,15s)-isomer
4. Flolan
5. Prostacyclin
6. Prostaglandin I(2)
7. Prostaglandin I2
8. Veletri
1. Flolan
2. Epoprostenol
3. Prostacyclin Sodium Salt
4. 61849-14-7
5. Sodium Prostacyclin
6. Prostaglandin I2
7. U-53217a
8. U-53,217a
9. 4k04iq1of4
10. Cyclo-prostin
11. 35121-78-9
12. Epoprostenol Sodium [usan:ban]
13. Ncgc00167427-01
14. Einecs 263-273-7
15. Unii-4k04iq1of4
16. Floran
17. U 53,217a
18. Epoprostenolsodium
19. Prostacyclin Sodium
20. Prostaglandin X Sodium
21. Prostaglandin I2 Na
22. Chembl962
23. Dsstox_cid_26617
24. Dsstox_rid_81769
25. Sodium (5z,13e,15s)-6,9alpha-epoxy-11alpha,15-dihydroxyprosta-5,13-dien-1-oate
26. Dsstox_gsid_46617
27. Schembl41344
28. Sodium (z)-(3ar,4r,5r,6as)-hexahydro-5-hydroxy-4-((e)-(3s)-3-hydroxy-1-octenyl)-2h-cyclopenta(b)furan-delta(sup 2,delta)-valerate
29. Bml1-f10
30. Dtxsid3046617
31. Epoprostenol Sodium [jan]
32. 4ua76
33. Epoprostenol Sodium [usan]
34. Hms1361k18
35. Hms3648l14
36. Epoprostenol Sodium [vandf]
37. Epoprostenol Sodium [mart.]
38. Tox21_112431
39. Epoprostenol Sodium [usp-rs]
40. Epoprostenol Sodium [who-dd]
41. Prostacyclin Sodium Salt [mi]
42. Akos024457370
43. Epoprostenol Sodium [orange Book]
44. Prosta-5,13-dien-1-oic Acid, 6,9-epoxy-11,15-dihydroxy-, Sodium Salt, (5z,9alpha,11alpha,13e,15s)-
45. Cas-61849-14-7
46. Q27259818
47. (5z,13e,8r,9s,11r,12r,15s)-6,9-epoxy-11,15-dihydroxyprosta-5,13-dien-1-oic Acid Sodium Salt
48. Prosta-5,13-dien-1-oic Acid, 6,9-epoxy-11,15-dihydroxy-, Sodium Salt, (5z,9.alpha.,11.alpha.,13e,15s)-
49. Sodium (z)-(3ar,4r,5r,6as)-hexahydro-5-hydroxy-4-((e)-(3s)-3-hydroxy-1-octenyl)-2h-cyclopenta(b)furan-.delta.(sup 2,.delta.)-valerate
50. Sodium (z)-5-((3ar,4r,5r,6as)-5-hydroxy-4-((s,e)-3-hydroxyoct-1-enyl)hexahydro-2h-cyclopenta[b]furan-2-ylidene)pentanoate
| Molecular Weight | 374.4 g/mol |
|---|---|
| Molecular Formula | C20H31NaO5 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 10 |
| Exact Mass | 374.20691837 g/mol |
| Monoisotopic Mass | 374.20691837 g/mol |
| Topological Polar Surface Area | 89.8 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 491 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Epoprostenol sodium |
| PubMed Health | Epoprostenol (Injection) |
| Drug Classes | Peripheral Vasodilator, Platelet Aggregation Inhibitor |
| Drug Label | Epoprostenol sodium for injection is a sterile sodium salt formulated for intravenous (IV) administration. Each vial of epoprostenol sodium for injection contains epoprostenol sodium equivalent to either 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) e... |
| Active Ingredient | Epoprostenol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1.5mg base/vial; eq 0.5mg base/vial |
| Market Status | Prescription |
| Company | Teva Pharms Usa |
| 2 of 4 | |
|---|---|
| Drug Name | Flolan |
| Drug Label | FLOLAN (epoprostenol sodium) for Injection is a sterile sodium salt formulated for intravenous (IV) administration. Each vial of FLOLAN contains epoprostenol sodium equivalent to either 0.5mg (500,000ng) or 1.5mg (1,500,000ng) epoprostenol, 3... |
| Active Ingredient | Epoprostenol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1.5mg base/vial; eq 0.5mg base/vial |
| Market Status | Prescription |
| Company | Glaxosmithkline |
| 3 of 4 | |
|---|---|
| Drug Name | Epoprostenol sodium |
| PubMed Health | Epoprostenol (Injection) |
| Drug Classes | Peripheral Vasodilator, Platelet Aggregation Inhibitor |
| Drug Label | Epoprostenol sodium for injection is a sterile sodium salt formulated for intravenous (IV) administration. Each vial of epoprostenol sodium for injection contains epoprostenol sodium equivalent to either 0.5 mg (500,000 ng) or 1.5 mg (1,500,000 ng) e... |
| Active Ingredient | Epoprostenol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1.5mg base/vial; eq 0.5mg base/vial |
| Market Status | Prescription |
| Company | Teva Pharms Usa |
| 4 of 4 | |
|---|---|
| Drug Name | Flolan |
| Drug Label | FLOLAN (epoprostenol sodium) for Injection is a sterile sodium salt formulated for intravenous (IV) administration. Each vial of FLOLAN contains epoprostenol sodium equivalent to either 0.5mg (500,000ng) or 1.5mg (1,500,000ng) epoprostenol, 3... |
| Active Ingredient | Epoprostenol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 1.5mg base/vial; eq 0.5mg base/vial |
| Market Status | Prescription |
| Company | Glaxosmithkline |
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
B - Blood and blood forming organs
B01 - Antithrombotic agents
B01A - Antithrombotic agents
B01AC - Platelet aggregation inhibitors excl. heparin
B01AC09 - Epoprostenol




