


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
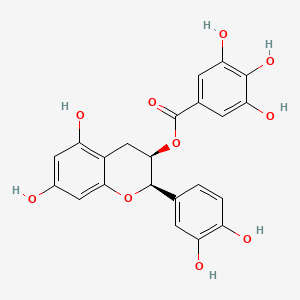

1. Epicatechin Gallate
2. Epicatechin Gallate, (2r-cis)-isomer
3. Epicatechin-3-gallate
4. Epicatechin-3-galloyl Ester
5. Epicatechin-3-o-gallate
1. 1257-08-5
2. Epicatechin Gallate
3. (-)-epicatechin-3-o-gallate
4. (-)-epicatechingallate
5. (-)-epicatechin-3-gallate
6. L-epicatechin Gallate
7. Epicatechin Monogallate
8. Teatannin
9. (-)-epicatechin 3-o-gallate
10. Ecg
11. Epicatechol, Gallate
12. 3-o-galloylepicatechin
13. (-)-cis-3,3',4',5,7-pentahydroxyflavane 3-gallate
14. [(2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2h-chromen-3-yl] 3,4,5-trihydroxybenzoate
15. 3-gallate(-)-epicatechol
16. (-)-epicatechin 3-gallate
17. Chembl36327
18. Epicatechin-3-o-gallate
19. Chebi:70255
20. 92587ovd8z
21. Epicatechin-3-galloyl Ester
22. (2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-3-yl 3,4,5-trihydroxybenzoate
23. Benzoic Acid, 3,4,5-trihydroxy-, (2r,3r)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7-dihydroxy-2h-1-benzopyran-3-yl Ester
24. Epicatechin 3-gallate
25. Epi-catechin 3-o-gallate
26. Epicatechin Gallate, (2r-cis)-isomer
27. Nsc 636594
28. Unii-92587ovd8z
29. Nsc636594
30. (2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2h-chromen-3-yl 3,4,5-trihydroxybenzoate
31. Epicatechol, 3-gallate, (-)-
32. Nsc-636594
33. Mfcd00075936
34. (-)epicatechingallate
35. Spectrum_000314
36. L-ecg
37. Specplus_000275
38. (-) Epicatechin Gallate
39. Epicatechin-gallate-(-)
40. Spectrum2_000165
41. Spectrum3_000246
42. Spectrum4_001540
43. Spectrum5_000080
44. (-)-cis-2-(3,4-dihydroxyphenyl)-3,4-dihydro-1(2h)-benzopyran-3,5,7-triol 3-gallate
45. (?)-epicatechin 3-gallate
46. Schembl39047
47. Bspbio_001632
48. Epicatechol 3-gallate
49. Kbiogr_001980
50. Kbioss_000794
51. Spectrum210238
52. Benzoic Acid, 3,4,5-trihydroxy-, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7-dihydroxy-2h-1-benzopyran-3-yl Ester, (2r-cis)-
53. Divk1c_006371
54. Epicatechingallate, L-
55. Spbio_000029
56. (-)epicatechin Gallate
57. Megxp0_000810
58. Epicatechin 3-o-gallate
59. Kbio1_001315
60. Kbio2_000794
61. Kbio2_003362
62. Kbio2_005930
63. Kbio3_001132
64. Dtxsid70925231
65. 3,4,5-trihydroxy-benzoic Acid 2-(3,4-dihydroxy-phenyl)-5,7-dihydroxy-chroman-3-yl Ester
66. Hy-n0002
67. Zinc3978503
68. Bdbm50153015
69. Ccg-38376
70. Lmpk12020090
71. S3925
72. Akos015965216
73. Ac-6037
74. Am84365
75. Cs-3761
76. Sdccgmls-0066549.p001
77. (-)-epi Catechin-3-o-gallate
78. [(2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-chroman-3-yl] 3,4,5-trihydroxybenzoate
79. Ncgc00179135-01
80. As-15722
81. E0890
82. 257e085
83. Sr-05000002675
84. Q-200002
85. Q5382492
86. Sr-05000002675-1
87. Brd-k50660797-001-01-0
88. Brd-k50660797-001-03-6
89. (-)-epicatechin Gallate, >=98% (hplc), From Green Tea
90. Epicatechin Gallate, Primary Pharmaceutical Reference Standard
91. [(2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-3-yl] 3,4,5-trihydroxybenzoate
92. Rel-(2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxychroman-3-yl 3,4,5-trihydroxybenzoate
93. (-)-epicatechin-3-o-gallate (constituent Of Grape Seeds Oligomeric Proanthocyanidins) [dsc]
94. (-)-epicatechin-3-o-gallate(egc) (constituent Of Powdered Decaffeinated Green Tea Extract) [dsc]
95. (2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2h-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate
96. 3,4,5-trihydroxy-benzoic Acid (2r,3r)-2-(3,4-dihydroxy-phenyl)-5,7-dihydroxy-chroman-3-yl Ester
97. Benzoic Acid, 3,4,5-trihydroxy-, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7- Dihydroxy-2h-1-benzopyran-3-yl Ester, (-)-cis-
98. Ncgc00179135-03![(2r,3r)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2h-chromen-3-yl] 3,4,5-trihydroxybenzoate
| Molecular Weight | 442.4 g/mol |
|---|---|
| Molecular Formula | C22H18O10 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 7 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 4 |
| Exact Mass | 442.08999677 g/mol |
| Monoisotopic Mass | 442.08999677 g/mol |
| Topological Polar Surface Area | 177 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 649 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Protease Inhibitors
Compounds which inhibit or antagonize biosynthesis or actions of proteases (ENDOPEPTIDASES). (See all compounds classified as Protease Inhibitors.)
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)


