



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
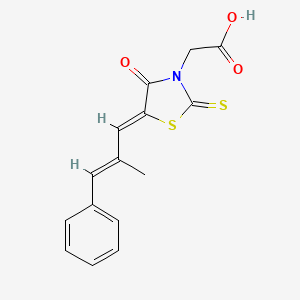

1. 3-carboxymethyl-5-(methyl-3-phenylpropenylidene)rhodanine
2. Ono 2
3. Ono 2235
4. Ono-2
5. Ono-2235
1. 82159-09-9
2. Kinedak
3. Epalrestatum
4. Epalrestat [inn]
5. Ono 2235
6. Ono-2235
7. Ono-2
8. 2-((z)-5-((e)-2-methyl-3-phenylallylidene)-4-oxo-2-thioxothiazolidin-3-yl)acetic Acid
9. Aldonil
10. Aldorin
11. Tanglin
12. 2-[(5z)-5-[(e)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]acetic Acid
13. Chembl56337
14. 5-((1z,2e)-2-methyl-3-phenylpropenylidene)-4-oxo-2-thioxo-3-thiazolidineacetic Acid
15. Chebi:31539
16. Ono2235
17. 5-((z,e)-beta-methylcinnamylidene)-4-oxo-2-thioxo-3-thiazolidineacetic Acid
18. Mfcd00865484
19. 424dv0807x
20. {(5z)-5-[(2e)-2-methyl-3-phenylprop-2-en-1-ylidene]-4-oxo-2-thioxo-1,3-thiazolidin-3-yl}acetic Acid
21. Epalrestatum [latin]
22. {5-[(e)-2-methyl-3-phenyl-prop-2-en-(z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl}-acetic Acid
23. 2-[(5z)-5-[(2e)-2-methyl-3-phenylprop-2-en-1-ylidene]-4-oxo-2-sulfanylidene-1,3-thiazolidin-3-yl]acetic Acid
24. Ono 2
25. Kinedak (tn)
26. Unii-424dv0807x
27. Ncgc00164613-01
28. Epalrestat- Bio-x
29. 5-[(1z, 2e)-2-methyl-3-phenylpropenylidene]-4-oxo2-thioxo-3-thiazolidineacetic Acid
30. Epalrestat [mi]
31. Epalrestat [jan]
32. Epalrestat (jp17/inn)
33. Epalrestat [mart.]
34. 3-thiazolidineacetic Acid, 5-(2-methyl-3-phenyl-2-propenylidene)-4-oxo-2-thioxo-, (e,e)-
35. Epalrestat [who-dd]
36. Schembl49049
37. Mls000806985
38. Dtxsid1046479
39. Gtpl11371
40. Regid_for_cid_1549120
41. Hms2747m09
42. Hms3887a17
43. Zinc1533688
44. Bbl029067
45. Bdbm50049730
46. S2035
47. Stk337187
48. Akos000274207
49. Bcp9000649
50. Ccg-267693
51. Db15293
52. Ncgc00164613-08
53. Ncgc00164613-12
54. As-13345
55. Be164412
56. Hy-66009
57. Smr000414799
58. Bcp0726000053
59. E0906
60. Sw219826-1
61. D01688
62. Ab00647195_06
63. 159e099
64. Q5382029
65. [5-(2-methyl-3-phenyl-allylidene)-4-oxo-2-thioxo-thiazolidin-3-yl]-acetic Acid
66. 2-(5-(2-methyl-3-phenylallylidene)-4-oxo-2-thioxothiazolidin-3-yl)acetic Acid
67. 5-((z,e)-.beta.-methylcinnamylidene)-4-oxo-2-thioxo-3-thiazolidineacetic Acid
68. (5-[(e)-2-methyl-3-phenyl-prop-2-en-(z)-ylidene]-4-oxo-2-thioxo-thiazolidin-3-yl)-acetic Acid 82159-
69. {(5z)-5-[(2e)-2-methyl-3-phenylprop-2-enylidene]-4-oxo-2-thioxo-1,3-thiazolidin-3-yl}acetic Acid
70. 2-[(5z)-5-[(e)-3-phenil-2-methylprop-2-enylidene]-4-oxo-2-thioxo-3-thiazolidinyl]acetic Acid
| Molecular Weight | 319.4 g/mol |
|---|---|
| Molecular Formula | C15H13NO3S2 |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | 319.03368562 g/mol |
| Monoisotopic Mass | 319.03368562 g/mol |
| Topological Polar Surface Area | 115 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 519 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 2 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Enzyme Inhibitors
Compounds or agents that combine with an enzyme in such a manner as to prevent the normal substrate-enzyme combination and the catalytic reaction. (See all compounds classified as Enzyme Inhibitors.)


