


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
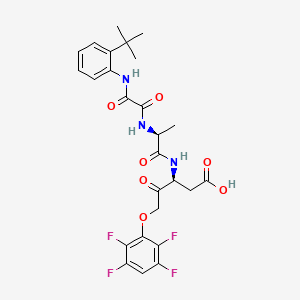

1. 3-(2-(2-tert-butylphenylaminooxalyl)aminopropionylamino)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
2. Idn 6556
3. Idn-6556
4. Idn6556
5. Pf 03491390
6. Pf-03491390
7. Pf03491390
1. 254750-02-2
2. Idn-6556
3. Pf-03491390
4. Pf 03491390
5. (s)-3-((s)-2-(2-(2-tert-butylphenylamino)-2-oxoacetamido)propanamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
6. Idn 6556
7. (s)-3-((s)-2-(2-((2-(tert-butyl)phenyl)amino)-2-oxoacetamido)propanamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
8. Vay785
9. Vay-785
10. P0gms9n47q
11. (3s)-3-[[(2s)-2-[[2-(2-tert-butylanilino)-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
12. L-alaninamide, N-(2-(1,1-dimethylethyl)phenyl)-2-oxoglycyl-n-((1s)-1-(carboxymethyl)-2-oxo-3-(2,3,5,6-tetrafluorophenoxy)propyl)-
13. Emricasan [usan]
14. Emricasan [usan:inn]
15. Unii-p0gms9n47q
16. C26h27f4n3o7
17. Emricasan [inn]
18. Emricasan(idn6556)
19. Emricasan (usan/inn)
20. Emricasan [who-dd]
21. Chembl197672
22. Gtpl6508
23. Schembl3288801
24. Dtxsid10180160
25. Amy36447
26. Bcp07463
27. Ex-a1659
28. Bdbm50461533
29. S7775
30. Zinc14191207
31. Akos016009462
32. Ccg-270089
33. Cs-0599
34. Db05408
35. Ncgc00346477-01
36. Ac-31507
37. As-75073
38. Hy-10396
39. Pf03491390
40. Emricasan(idn6556,pf03491390)
41. J3.566.323a
42. D10004
43. Emricasan (idn-6556, Pf-03491390)
44. A857136
45. Q27077178
46. (3s)-3-((2s)-2-((n-(2-tert-butyl)phenyl)carbamoyl)carbonylamino) Propanoylamino)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
47. (3s)-3-(n2-((2-tert-butylphenyl)oxamoyl)-l-alaninamido)-4-oxo-5-(2,3,5,6- Tetrafluorophenoxy)pentanoic Acid
48. (3s)-3-(n2-((2-tert-butylphenyl)oxamoyl)-l-alaninamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
49. (3s)-3-[(2s)-2-{[(2-tert-butylphenyl)carbamoyl]formamido}propanamido]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
50. (3s)-3-[[(2s)-2-[[2-(2-tert-butylanilino)-2-oxo-acetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
51. (3s)-3-[[(2s)-2-[[2-[(2-tert-butylphenyl)amino]-2-oxoacetyl]amino]propanoyl]amino]-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoic Acid
52. (s)-3-((s)-2-(2-((2-(tert-butyl)phenyl)amino)-2-oxoacetamido)propanamido)-4-oxo-5-(2,3,5,6-tetrafluorophenoxy)pentanoicacid
| Molecular Weight | 569.5 g/mol |
|---|---|
| Molecular Formula | C26H27F4N3O7 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 11 |
| Exact Mass | 569.17851286 g/mol |
| Monoisotopic Mass | 569.17851286 g/mol |
| Topological Polar Surface Area | 151 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 934 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in hepatitis (viral, C), liver disease, and transplantation (organ or tissue).
Treatment of non-alcoholic steatohepatitis (NASH)
IDN-6556 significantly improves markers of liver damage in patients infected with the Hepatitis C virus (HCV), an infection that affects up to 170m patients worldwide. IDN-6556 represents a new class of drugs that protect the liver from inflammation and cellular damage induced by viral infections and other causes. Various studies have also shown that it significantly lowers aminotransferase activity in HCV patients and appeared to be well tolerated.


