



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
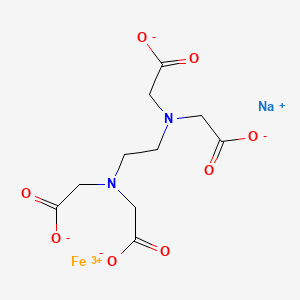

1. Ammonium Ferric Edetate
2. Edta Fe(iii)
3. Edta Ferric Ammonium
4. Fe(iii)-edta
5. Fe(iii)-edta Complex (1:1)
6. Fe(iii)-edta, Ammonium Salt
7. Fe(iii)-edta, Potassium Salt
8. Fe(iii)-edta, Sodium Salt
9. Fe(iii)-edta, Sodium Salt, Trihydrate
10. Ferrate(1-), ((ethylenedinitrilo)tetraacetato)-, Hydrogen
11. Ferric Edta
12. Ferric Sodium Edetate
13. Ferric-edta
14. Hydrogen ((ethylenedinitrilo)tetraacetato)ferrate(iii)
15. Iron(iii) Edta
16. Irostrene
17. Monoferric Edetate
18. Nafeedta
19. Sodium Iron Edta
20. Sytron
1. 15708-41-5
2. Edta Ferric Sodium Salt
3. Edta Ferric-sodium Salt
4. Edta Iron(iii) Sodium Salt
5. Ferric Sodium Edta
6. 18154-32-0
7. Sodium Iron Edta
8. Sytron
9. Ethylenediaminetetraacetic Acid Ferric Sodium Salt
10. Ethylenediaminetetraacetic Acid Iron(iii) Sodium Salt
11. Sodium Iron Edetate
12. Ethylenediaminetetraacetic Acid,monosodium Ferric Salt
13. Ferric Sodium Ethylenediaminetetraacetate
14. Sybron
15. Sodium;2-[2-[bis(carboxylatomethyl)amino]ethyl-(carboxylatomethyl)amino]acetate;iron(3+)
16. 157009-77-3
17. Ncgc00164429-01
18. 9,12-octadecadienoic Acid (9z,12z)-, Dimer, Diisoeicosyl Ester
19. Iron(iii) Sodium Ethylenediaminetetraacetate
20. Sodium Feredetate (inn)
21. Dsstox_cid_7774
22. Dsstox_rid_78564
23. Dsstox_gsid_27774
24. Sodium Feredetate [inn]
25. Ferrazone
26. 403j23emfa
27. Cas-15708-41-5
28. Nsc-5237
29. Iron Chelate Of The Monosodium Salt Of (ethylenedinitrilo)tetraacetic Acid
30. Mfcd00078215
31. Edta, Iron (iii) Sodium
32. Sytron (tn)
33. Iron(3+) Sodium 2,2',2'',2'''-(ethane-1,2-diyldinitrilo)tetraacetate
34. Diisoarachidyl Dilinoleate
35. Edetic Acid Sodium Iron Salt
36. Schembl65157
37. Sodium Iron Edta [fcc]
38. Dtxsid5027774
39. Sodium Feredetate [mart.]
40. Ferric Sodium Edetate [mi]
41. Sodium Feredetate [who-dd]
42. Sodium Iron Edta [usp-rs]
43. Tox21_112117
44. Tox21_201923
45. Tox21_303443
46. Akos015903831
47. Akos015914864
48. Tox21_112117_1
49. Ncgc00159485-06
50. Ncgc00164429-02
51. Ncgc00257487-01
52. Ncgc00259472-01
53. Ferrate(1-), [[n,n'-1,2-ethanediylbis[n-[(carboxy-.kappa.o)methyl]glycinato-.kappa.n,.kappa.o]](4-)]-, Sodium, (oc-6-21)-
54. E0092
55. Ft-0658850
56. Ft-0689141
57. D07145
58. F71420
59. Sodium Iron Ethylenediamine Tetraacetic Acid
60. Iron(iii) Sodium Ethylenediamine Tetraacetate
61. Iron(iii)-sodium Ethylenediaminetetraacetate Hydrate
62. J-008546
63. Ethylenediaminetetraacetic Acid Monosodium Ferric Salt
64. Q30314945
65. Ethylenediaminetetraacetic Acid, Iron(iii) Monosodium Salt
66. [1,2-ethanediylbis(nitrilo)]tetraacetic Acid 1,1',1''-iron(iii)1'''-sodium Salt
67. Iron(3+) Sodium 2,2',2'',2'''-(ethane-1,2-diyldinitrilo)tetraacetate (1:1:1)
68. Edta Ferric Sodium Salt;edathamil; Edta Iron(iii) Sodium Salt; Ethylenediaminetetraacetic Acid Ferric Sodium Salt
69. Ferrate(1-), [[n,n'-1,2-ethanediylbis[n-[(carboxy-.kappa.o)methyl]glycinato-.kappa.n,.kappa.o]](4-)]-, Sodium (1:1), (oc-6-21)-
| Molecular Weight | 367.05 g/mol |
|---|---|
| Molecular Formula | C10H12FeN2NaO8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 7 |
| Exact Mass | 366.984070 g/mol |
| Monoisotopic Mass | 366.984070 g/mol |
| Topological Polar Surface Area | 167 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 293 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Iron Chelating Agents
Organic chemicals that form two or more coordination links with an iron ion. Once coordination has occurred, the complex formed is called a chelate. The iron-binding porphyrin group of hemoglobin is an example of a metal chelate found in biological systems. (See all compounds classified as Iron Chelating Agents.)
B - Blood and blood forming organs
B03 - Antianemic preparations
B03A - Iron preparations
B03AB - Iron trivalent, oral preparations
B03AB03 - Sodium feredetate


