
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
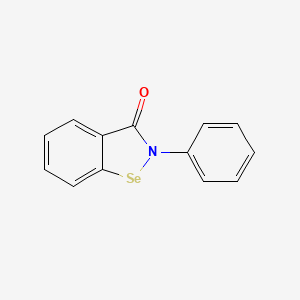

1. 2-phenyl-1,2-benzoisoselenazol-3(2h)-one
2. 2-phenylbenzoisoselenazol-3(2h)-one
3. Dr 3305
4. Dr-3305
5. Dr3305
6. Pz 51
7. Pz-51
8. Rp 60931
9. Spi 1005
10. Spi-1005
11. Spi1005
1. 60940-34-3
2. 2-phenyl-1,2-benzisoselenazol-3(2h)-one
3. 2-phenylbenzo[d][1,2]selenazol-3(2h)-one
4. Ebselenum
5. 2-phenyl-1,2-benzoselenazol-3-one
6. Spi-1005
7. Ebselene
8. Ebseleno
9. Ebselen [inn]
10. Ebselene [french]
11. Ebselenum [latin]
12. Harmokisane
13. Ebseleno [spanish]
14. Pz 51
15. Dr-3305
16. Pz-51
17. Pz51
18. Mls000028488
19. Dr3305
20. Ccris 3714
21. 1,2-benzisoselenazol-3(2h)-one, 2-phenyl-
22. 2-phenyl-1,2-benzisoselenazolin-3-one
23. Smr000058445
24. Unii-40x2p7dpgh
25. 2-phenyl-1,2-benzoisoselenazol-3(2h)-one
26. Chembl51085
27. Nsc 639762
28. Prestwick_1057
29. Spi-3005
30. Prestwick0_000740
31. Prestwick1_000740
32. Prestwick2_000740
33. Prestwick3_000740
34. Spectrum2_001441
35. Spectrum3_000799
36. Spectrum4_000445
37. Spectrum5_001713
38. Lopac-e-3520
39. Mfcd00210937
40. Nsc639762
41. Nsc-639762
42. Nsc-757883
43. 40x2p7dpgh
44. Lopac0_000541
45. Ncgc00015412-06
46. Bspbio_000700
47. Bspbio_001342
48. Bspbio_002538
49. Cpd000058445
50. Kbiogr_000062
51. Kbiogr_000830
52. Kbioss_000062
53. Divk1c_000951
54. Spbio_001301
55. Spbio_002639
56. Cas-60940-34-3
57. Mls001148646
58. Bpbio1_000770
59. Bcbcmap01_000149
60. Chebi:77543
61. Hms502p13
62. Kbio1_000951
63. Kbio2_000062
64. Kbio2_002630
65. Kbio2_005198
66. Kbio3_000123
67. Kbio3_000124
68. Kbio3_001758
69. Ninds_000951
70. E 3520
71. Bio2_000062
72. Bio2_000542
73. Hms1361d04
74. Hms1570c22
75. Hms1791d04
76. Hms1989d04
77. Hms2052n09
78. Ccg-39161
79. Ac-1124
80. C13h9nose
81. Idi1_000951
82. Idi1_033812
83. Qtl1_000035
84. Ncgc00015412-01
85. Ncgc00015412-02
86. Ncgc00015412-03
87. Ncgc00015412-13
88. Ncgc00024072-03
89. Ncgc00024072-04
90. Ncgc00024072-05
91. Ncgc00178610-01
92. Ncgc00178610-02
93. Ncgc00178610-03
94. Ab00053217
95. Eu-0100541
96. Mls-0003066.0001
97. Brd-k29359156-001-06-1
98. Dr 3305
99. Rp 60931
100. Sr-01000003081
101. Ac1l1fdw
102. Cid3194
103. Spi1005
104. Ebselen (c5)
105. Nchembio.109-comp1
106. 2-phenyl-1,2-benzoselenazol-3(2h)-one
107. Ls-33527
108. Sam001247071
109. Ebselen [jan]
110. Ebselen [mi]
111. Ebselen [mart.]
112. Opera_id_1643
113. Ebselen [who-dd]
114. Ebselen, Cysteine Modifier
115. Cid_3194
116. Dsstox_cid_25150
117. Dsstox_rid_80704
118. C042986
119. Dsstox_gsid_45150
120. Schembl33829
121. Mls001424261
122. Mls006010108
123. E3520_sigma
124. I09-1611
125. Dtxsid7045150
126. Bdbm34233
127. Gtpl10583
128. Hms2097c22
129. Hms2235a11
130. Hms3394n09
131. Hms3402d04
132. Hms3649o05
133. Hms3714c22
134. Hms3873n13
135. Kuc112559n
136. Pharmakon1600-01501188
137. Bcp17134
138. Ex-a1447
139. Spi-1005;pz-51
140. Tox21_110140
141. 2-phenyl-benzo[d]isoselenazol-3-one
142. Dap001372
143. Nsc757883
144. S6676
145. Akos015898841
146. Cs-5534
147. Db12610
148. Lp00541
149. Nc00431
150. Sdccgsbi-0050524.p004
151. Ksc-325-014
152. Ncgc00015412-04
153. Ncgc00015412-05
154. Ncgc00015412-07
155. Ncgc00015412-08
156. Ncgc00015412-09
157. Ncgc00015412-10
158. Ncgc00015412-11
159. Ncgc00015412-12
160. Ncgc00015412-21
161. Phenyl-1,2-benzisoselenazol-3(2h)-one
162. 2-phenyl-1,2-benzisoselazol-3(2h)-one
163. Hy-13750
164. Sy052687
165. Sbi-0050524.p003
166. 2-phenyl-1,2-benzisoselenazole-3(2h)-one
167. Db-072873
168. 2-phenyl-1,2-benzoisoselenazole-3(2h)-one
169. E0946
170. Ft-0759332
171. 2-phenyl-1,2-benzoisoselenazole-3-(2h)-one
172. 2-phenyl-benzo[d]isoselenazol-3-one(ebselen)
173. C75847
174. Ab00053217_25
175. 940e343
176. A868855
177. Q5332073
178. Sr-01000003081-2
179. Sr-01000003081-7
180. Sr-01000003081-8
181. Brd-k29359156-001-23-6
182. Sr-01000003081-10
183. Sr-01000003081-14
| Molecular Weight | 274.19 g/mol |
|---|---|
| Molecular Formula | C13H9NOSe |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 1 |
| Exact Mass | 274.98494 g/mol |
| Monoisotopic Mass | 274.98494 g/mol |
| Topological Polar Surface Area | 20.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 275 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Anti-Ulcer Agents
Various agents with different action mechanisms used to treat or ameliorate PEPTIC ULCER or irritation of the gastrointestinal tract. This has included ANTIBIOTICS to treat HELICOBACTER INFECTIONS; HISTAMINE H2 ANTAGONISTS to reduce GASTRIC ACID secretion; and ANTACIDS for symptomatic relief. (See all compounds classified as Anti-Ulcer Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
Neuroprotective Agents
Drugs intended to prevent damage to the brain or spinal cord from ischemia, stroke, convulsions, or trauma. Some must be administered before the event, but others may be effective for some time after. They act by a variety of mechanisms, but often directly or indirectly minimize the damage produced by endogenous excitatory amino acids. (See all compounds classified as Neuroprotective Agents.)
ABOUT THIS PAGE
49
PharmaCompass offers a list of Ebselen API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Ebselen manufacturer or Ebselen supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Ebselen manufacturer or Ebselen supplier.
PharmaCompass also assists you with knowing the Ebselen API Price utilized in the formulation of products. Ebselen API Price is not always fixed or binding as the Ebselen Price is obtained through a variety of data sources. The Ebselen Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Ebselen manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Ebselen, including repackagers and relabelers. The FDA regulates Ebselen manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Ebselen API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Ebselen supplier is an individual or a company that provides Ebselen active pharmaceutical ingredient (API) or Ebselen finished formulations upon request. The Ebselen suppliers may include Ebselen API manufacturers, exporters, distributors and traders.
Ebselen Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Ebselen GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Ebselen GMP manufacturer or Ebselen GMP API supplier for your needs.
A Ebselen CoA (Certificate of Analysis) is a formal document that attests to Ebselen's compliance with Ebselen specifications and serves as a tool for batch-level quality control.
Ebselen CoA mostly includes findings from lab analyses of a specific batch. For each Ebselen CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Ebselen may be tested according to a variety of international standards, such as European Pharmacopoeia (Ebselen EP), Ebselen JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Ebselen USP).

