



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada

Australia

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
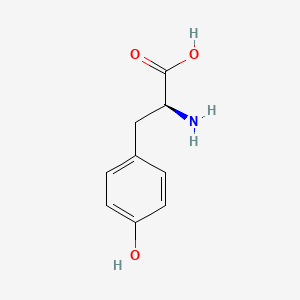

1. L Tyrosine
2. L-tyrosine
3. Para Tyrosine
4. Para-tyrosine
5. Tyrosine, L Isomer
6. Tyrosine, L-isomer
1. L-tyrosine
2. 60-18-4
3. (s)-tyrosine
4. P-tyrosine
5. L-p-tyrosine
6. (2s)-2-amino-3-(4-hydroxyphenyl)propanoic Acid
7. H-tyr-oh
8. 4-hydroxy-l-phenylalanine
9. Tyrosine, L-
10. Tyrosinum [latin]
11. Tyrosine (van)
12. Tirosina [spanish]
13. L-(-)-tyrosine
14. (-)-alpha-amino-p-hydroxyhydrocinnamic Acid
15. L-phenylalanine, 4-hydroxy-
16. Beta-(p-hydroxyphenyl)alanine
17. Tyrosine [usan:inn]
18. Fema No. 3736
19. (s)-alpha-amino-4-hydroxybenzenepropanoic Acid
20. L-2-amino-3-p-hydroxyphenylpropanoic Acid
21. 3-(4-hydroxyphenyl)-l-alanine
22. (s)-3-(p-hydroxyphenyl)alanine
23. (s)-(-)-tyrosine
24. Hsdb 2003
25. Alpha-amino-beta-(4-hydroxyphenyl)propionic Acid
26. Ai3-09055
27. Tirosina
28. Propanoic Acid, 2-amino-3-(4-hydroxyphenyl)-, (s)-
29. Nsc 82624
30. Alpha-amino-p-hydroxyhydrocinnamic Acid, (-)-
31. L-tyr
32. (s)-2-amino-3-(4-hydroxyphenyl)propanoic Acid
33. (s)-2-amino-3-(p-hydroxyphenyl)propionic Acid
34. L-tyrosine, Monomer
35. Alpha-amino-4-hydroxybenzenepropanoic Acid, (s)-
36. 2-amino-3-(4-hydroxyphenyl)propanoic Acid, (s)-
37. Benzenepropanoic Acid, Alpha-amino-4-hydroxy-, (s)-
38. Mfcd00002606
39. Tyr
40. 25619-78-7
41. Chebi:17895
42. Nsc-82624
43. 42hk56048u
44. Ncgc00159350-02
45. Tyrosine (l-tyrosine)
46. Tyrosinum
47. Dsstox_cid_3730
48. L-tyrosin
49. (-)-.alpha.-amino-p-hydroxyhydrocinnamic Acid
50. Dsstox_rid_77170
51. Dsstox_gsid_23730
52. L-tyrosine, Homopolymer
53. Cas-60-18-4
54. L-tyrosine (9ci)
55. 4ts1
56. Tyrosine (usp/inn)
57. Einecs 200-460-4
58. Plovamer-acetate
59. Benzenepropanoate
60. Unii-42hk56048u
61. 2csm
62. (s)-2-amino-3-(4-hydroxyphenyl)propionic Acid
63. (l)-tyrosine
64. (-) Tyrosine
65. H-tyr
66. L-tyrosine,(s)
67. L-tyrosine (jp17)
68. Tyrosine [hsdb]
69. Tyrosine [inci]
70. Tyrosine [usan]
71. Tyrosine [inn]
72. Tyrosine [ii]
73. Tyrosine [mi]
74. L-tyr-oh
75. Tyrosine [vandf]
76. Tyrosine, L- (8ci)
77. .alpha.-amino-.beta.-(4-hydroxyphenyl)propionic Acid
78. L-tyrosine [fcc]
79. L-tyrosine [jan]
80. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs
81. Tyrosine [mart.]
82. Bmse000051
83. Chembl925
84. L-tyrosine [fhfi]
85. Tyrosine [who-dd]
86. Schembl1581
87. L-[u-14c]tyr
88. L-phenylalanine-4-hydroxy-
89. L-tyrosine Non-animal Source
90. L-tyrosine [usp-rs]
91. Levodopa Impurity, L-tyrosine-
92. Tyrosine [orange Book]
93. Gtpl4791
94. L-tyrosine, >=97%, Fg
95. Tyrosine [ep Monograph]
96. Dd69927c-c6a8-4bc6-8e9a-0ab423b176e7
97. Dtxsid1023730
98. Tyrosine [usp Monograph]
99. Bdbm18129
100. Zinc266964
101. N-acetyl-o-(dihydroxymethylsilyl)-
102. Hy-n0473
103. L-tyrosine, Vetec(tm), 98.5%
104. Levodopa Related Compound L-tyrosine
105. Tox21_111594
106. (-)-a-amino-p-hydroxyhydrocinnamate
107. Ac2634
108. S4608
109. Akos010400205
110. Tox21_111594_1
111. (s)-a-amino-4-hydroxybenzenepropanoate
112. Am82304
113. Cs-8013
114. Db00135
115. (-)-alpha-amino-p-hydroxyhydrocinnamate
116. (-)-a-amino-p-hydroxyhydrocinnamic Acid
117. (s)-3-(4-hydroxyphenyl)alanine
118. (s)-a-amino-4-hydroxy-benzenepropanoate
119. Ncgc00159350-03
120. Ncgc00344525-01
121. Ac-11295
122. As-11772
123. Bp-13285
124. Levodopa Impurity B [ep Impurity]
125. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs, Oxidized With Hydrogen Peroxide
126. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase For 6 Hrs, Oxidized With Hydrogen Peroxide, <3 Kd Fraction
127. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase, Brominated With N-bromosuccinimide
128. Melanin Synthesized From Tyr Substrate Catalyzed By Tyrosinase, Sulfonated Using Sulfur Trioxide/dmf Complex For 1.5-7 Hours
129. (s)-alpha-amino-4-hydroxybenzenepropanoate
130. Diethyl1,3,5-benzenetricarboxylate
131. L-tyrosine, Bioultra, >=99.0% (nt)
132. (s)-2-amino-3-(p-hydroxyphenyl)propionate
133. (s)-a-amino-4-hydroxybenzenepropanoic Acid
134. Db-029987
135. L-tyrosine, Free Base - Cas 60-18-4
136. (s)-a-amino-4-hydroxy-benzenepropanoic Acid
137. (s)-alpha-amino-4-hydroxy-benzenepropanoate
138. L-tyrosine, Reagent Grade, >=98% (hplc)
139. L-tyrosine, Saj Special Grade, >=99.0%
140. T0550
141. C00082
142. D00022
143. D70837
144. L-tyrosine, Vetec(tm) Reagent Grade, >=98%
145. M02963
146. (2s)-2-amino-3-(4-hydroxyphenyl)propanoicacid
147. (s)-alpha-amino-4-hydroxy-benzenepropanoic Acid
148. L-tyrosine, Cell Culture Reagent (h-l-tyr-oh)
149. (s)-.alpha.-amino-4-hydroxybenzenepropanoic Acid
150. 002t606
151. 2-amino-3-(4-hydroxyphenyl)propanoic Acid-(s)-
152. A832631
153. N-acetyltyrosine Impurity A [ep Impurity]
154. Q188017
155. J-521656
156. Propanoic Acid, 2-amino-3-(4-hydroxyphenyl)-(s)-
157. Levodopa Impurity, L-tyrosine- [usp Impurity]
158. Q27115106
159. Benzenepropanoic Acid, .alpha.-amino-4-hydroxy-, (s)-
160. F8889-8713
161. L-tyrosine, Certified Reference Material, Tracecert(r)
162. Tyrosine, European Pharmacopoeia (ep) Reference Standard
163. Z1250208672
164. L-tyrosine, United States Pharmacopeia (usp) Reference Standard
165. 2-amino-3-(4-hydroxyphen Yl)-2-amino-3-(4-hydroxyphenyl)-propanoate
166. 2-amino-3-(4-hydroxyphen Yl)-2-amino-3-(4-hydroxyphenyl)-propanoic Acid
167. Benzeneethanaminium,a-carboxy-4-hydroxy-n,n,n-trimethyl-,inner Salt,(as)-
168. Benzeneethanaminium, A-carboxy-4-hydroxy-n,n,n-trimethyl-,inner Salt, (as)-
169. 1189756-47-5
170. L-tyrosine, From Non-animal Source, Meets Ep, Usp Testing Specifications, Suitable For Cell Culture, >=99.0%
171. L-tyrosine, Pharmagrade, Ajinomoto, Ep, Jp, Usp, Manufactured Under Appropriate Gmp Controls For Pharma Or Biopharmaceutical Production, Suitable For Cell Culture
| Molecular Weight | 181.19 g/mol |
|---|---|
| Molecular Formula | C9H11NO3 |
| XLogP3 | -2.3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 181.07389321 g/mol |
| Monoisotopic Mass | 181.07389321 g/mol |
| Topological Polar Surface Area | 83.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 176 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Tyrosine is claimed to act as an effective antidepressant, however results are mixed. Tyrosine has also been claimed to reduce stress and combat narcolepsy and chronic fatigue, however these claims have been refuted by some studies.
Tyrosine is a nonessential amino acid synthesized in the body from phenylalanine. Tyrosine is critical for the production of the body's proteins, enzymes and muscle tissue. Tyrosine is a precursor to the neurotransmitters norepinephrine and dopamine. It can act as a mood elevator and an anti-depressant. It may improve memory and increase mental alertness. Tyrosine aids in the production of melanin and plays a critical role in the production of thyroxin (thyroid hormones). Tyrosine deficiencies are manifested by hypothyroidism, low blood pressure and low body temperature. Supplemental tyrosine has been used to reduce stress and combat narcolepsy and chronic fatigue.
Absorption
L-tyrosine is absorbed from the small intestine by a sodium-dependent active transport process.
Semi-chronic exposure of ICR male Mice to Aflatoxin B1 in non-toxic doses results in elevated lung tryptophan levels without change in serotonin or 5-hydroxyindole-3-acetic acid levels. This change is organ specific in that tryptophan levels are not altered in spleen, duodenum, heart or central nervous system. Acute (48 hr) flunixin treatment decreases lung tryptophan levels and reverses the Aflatoxin B1 mediated increase in lung tryptophan levels. On the other hand, flunixin treatment decreases central nervous system tryptophan levels in control mice but not in Aflatoxin B1 treated mice. Aflatoxin B1 treated mice have an increase in splenic serotonin content. Acute (48 hr) treatment of mice with E. coli lipopolysaccharide also increases splenic serotonin, and Aflatoxin B1 treatment followed by lipopolysaccharide have a slightly additive effect on spleen serotonin content. Treatment of mice with lipopolysaccharide increases heart serotonin, an effect which is not altered in Aflatoxin B1 pretreated mice. Both lipopolysaccharide and Aflatoxin B1 per se increases lung tyrosine levels although the combination of treatments is not significantly different from the control value. Flunixin treatment increases lung tyrosine levels, an effect which is not altered by Aflatoxin B1 pretreatment. Acute treatment with either lipopolysaccharide or flunixin decreases the central nervous system tryptophan/tyrosine ratio; pretreatment with Aflatoxin B1 prevents those changes in the central nervous system tryptophan/tyrosine ratio. Central nervous system catecholamines are reduced in Aflatoxin B1 pretreated mice. However, central nervous system catecholamine changes in Aflatoxin B1 treated mice are normalized by vitamin E supplementation during the treatment period.
PMID:1906975 Weekley LB; Metab Brain Dis 6 (1): 19-32 (1991)
Male Wistar rats were divided in free choice conditions into heavy-drinkers consuming greater than 3.5 g/kg of ethanol daily, and light-drinkers consuming less than 2.0 g/kg/day. Subsequent 30 day intragastric administration of 25% ethanol (8-11 g/kg/day) caused an increase in permeability of the blood brain barrier to 14(C)-tyrosine, 14(C)-tryptophan and 14(C)-DOPA at all the stages of alcoholization. All the changes were more pronounced in light-drinkers than in heavy- drinker rats. Disulfiram, and to a lesser extent phenazepam and diazepam, when repeatedly injected (for 16-30 days) together with ethanol aggravated its effects.
PMID:2118321 Borisenko SA; Ann Ist Super Sanita 26 (1): 39-42 (1990)
Effects of mercury chloride (100 uM) para-chloromercuribenzene sulfonate (1 uM), and oxophenylarsine (250 uM) were determined on (a) the rate of sodium pump activity in intact winter flounder intestine; (b) activity of sodium potassium ATPase in tissue homogenates; and (c) sodium-dependent and sodium independent uptake of tyrosine in brush border membrane vesicles. All three agents decreased cell potassium, although effects on cell potassium lagged behind those for inhibition of the ATPase. At the concentrations used in the Ussing chamber (or at one-tenth concentration), all agents completely inhibited sodium potassium ATPase activity in enzyme assays performed with tissue homogenates. In contrast, only mercury chloride decreased sodium dependent uptake of tyrosine by brush border membrane vesicles. These results suggest that mercurial and arsenical effects on tyrosine absorption are due to inhibition of the sodium potassium ATPase thus decreasing the driving force for the cellular uptake by the sodium tyrosine cotransport system. Direct effects on sodium tyrosine cotransport may play a role in the inhibition observed with mercury chloride, but not for para-chloromercuribenzene sulfonate or oxophenylarsine.
PMID:2163123 Musch MW et al; Toxicol Appl Pharmacol 104 (1): 59-66 (1990)
Female Sprague-Dawley rats were treated acutely (12-hr) with aflatoxin B1 (100 ug/kg ip) or vehicle (10% acetone in 0.9% sodium chloride) and regional brain levels of tryptophan, serotonin and tyrosine were assayed. Brainstem but not cerebellar or cortical tyrosine levels were decreased in aflatoxin B1-treated rats. Brain tryptophan was increased in all 3 brain regions by acute aflatoxin B1 treatment, while serotonin levels were unaltered in the cerebellum and cortex and decreased in the brainstem. These experiments indicate that acute aflatoxin B1 treatment differentially alters brain amino acids and serotonin and that changes in brain tryptophan, the serotonin precursor, do not parallel changes in brain serotonin.
PMID:2500755 Weekley LB et al; Toxicol Lett 47 (2): 173-7 (1989)
For more Absorption, Distribution and Excretion (Complete) data for L-TYROSINE (10 total), please visit the HSDB record page.
In the liver, L-tyrosine is involved in a number of biochemical reactions, including protein synthesis and oxidative catabolic reactions. L-tyrosine that is not metabolized in the liver is distributed via the systemic circulation to the various tissues of the body.
/METABOLIC PATHWAY FOR L-TYROSINE:/ /TYROSINE GIVES/ P-HYDROXYPHENYLPYRUVIC ACID GIVES CO2 + HOMOGENTISIC ACID GIVES MALEYLACETOACETIC ACID GIVES FUMARYLACETOACETIC ACID GIVES FUMARATE + ACETOACETATE; TYROSINE GIVES 3,4-DIHYDROXYPHENYLALANINE GIVES CO2 + 3,4-DIHYDROXYPHENYLETHYLAMINE GIVES NORADRENALIN GIVES ADRENALIN.
Fenaroli's Handbook of Flavor Ingredients. Volume 2. Edited, translated, and revised by T.E. Furia and N. Bellanca. 2nd ed. Cleveland: The Chemical Rubber Co., 1975., p. 832
L-TYROSINE GIVES N-ACETYL-L-TYROSINE IN MAN; GIVES 3-CARBOXY-L-TYROSINE IN RESEDA; GIVES P-COUMARIC ACID IN SUGAR CANE, L-TYROSINE GIVES PARA-CRESOL IN PROTEUS; GIVES 3,4-DIHYDROXY-L-PHENYLALANINE IN HAMSTER; GIVES 3,4-DIHYDROXYSTILBENE-2-CARBOXYLIC ACID IN HYDRANGEA, L-TYROSINE GIVES 2,7-DIMETHYLNAPHTHOQUINONE IN CHIMAPHILA; GIVES L-DITYROSINE IN BEEF; GIVES PARA-HYDROXYMANDELONITRILE IN SORGHUM, L-TYROSINE GIVES PARA-HYDROXYPHENYLACETALDOXIME IN AUBRETIA; GIVES PARA-HYDROXYPHENYLPYRUVIC ACID IN RAT; GIVES 3-IODO-L-TYROSINE IN BEEF; L-TYROSINE GIVES LACHNANTHOSIDE IN LACHNANTHES; LOPHOCERINE IN LOPHOCERUS; MESEMBRINE IN SCELETIUM; NARWEDINE IN DAFFODIL, L-TYROSINE GIVES NOVOBIOCIN IN STREPTOMYCES; PHENOL IN RAT; BETA-TOCOPHEROL IN ANABAENA; TYLOPHORINE IN TYLOPHORA, L-TYROSINE GIVES TYRAMINE IN RAT; GIVES BETA-TYROSINE IN BACILLUS; GIVES L-TYROSINE HYDROXAMATE IN BEEF. L-TYROSINE GIVES L-TYROSINE-4-PHOSPHATE IN FLY; GIVES XANTHOCILLIN IN PENICILLIUM. /FROM TABLE/
Goodwin, B.L. Handbook of Intermediary Metabolism of Aromatic Compounds. New York: Wiley, 1976., p. T-47
Metabolism of tyrosine was impaired after chronic alcoholization of rats with 10% ethanol within 10 months. Within the first 3-4 months activation of tyrosine aminotransferase and a decrease in phenylalanine hydroxylase activity were found in liver tissue. Activity of tyrosine aminotransferase was not increased during the long term alcohol intoxication. At the same time, activity of tyrosine aminotransferase was decreased within 5-6 months simultaneously with activation of phenylalanine hydroxylase. An increase in the alcohol dehydrogenase activity was also observed in rat liver tissue during the initial period of intoxication. The enzymatic activity was decreased beginning from the 3-4 months of the alcoholization and maintained at the low level. Hyperthermia augmented these alterations observed in chronic alcoholization of rats.
Kurbanov KhK, Romakh ON; Vopr Med Khim 35 (4): 102-5 (1989)
Spontaneous behavior subsequent to acute oral administration of high doses of aspartame, phenylalanine, or tyrosine was analyzed using a computer pattern recognition system. Spraque Dawley male rats (250-300 g) were dosed orally with aspartame (500 or 100 mg/kg), phenylalanine (281 or 562 mg/kg), or tyrosine (309 or 618 mg/kg), and their behavior was analyzed 1 hr after dosing. The computer pattern recognition system recorded and classifed 13 different behavioral acts performed by the animals during the first 15-min exploration of a novel environment. These doses of aspartame, phenylalanine, and tyrosine did not induce any significant changes in spontaneous behavior. Unlike low doses of amphetamine and despite high plasma concentrations of phenylalanine and tyrosine, no behavioral alteration was detected by the computer pattern recognition system.
PMID:1677339 Mullenix PJ et al; Fundam Appl Toxicol 16 (3): 495-505 (1991)
For more Metabolism/Metabolites (Complete) data for L-TYROSINE (7 total), please visit the HSDB record page.
Tyrosine is produced in cells by hydroxylating the essential amino acid phenylalanine. This relationship is much like that between cysteine and methionine. Half of the phenylalanine required goes into the production of tyrosine; if the diet is rich in tyrosine itself, the requirements for phenylalanine are reduced by about 50%. The mechanism of L-tyrosine's antidepressant activity can be accounted for by the precursor role of L-tyrosine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects.


