



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
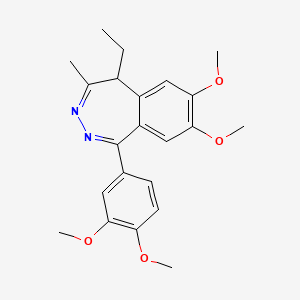

1. 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine
2. Dextofisopam
3. Egyt-341
4. Grandaxin
5. Levotofisopam
6. Tofizopam
1. 22345-47-7
2. Grandaxin
3. 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine
4. Emandaxin
5. Egyt 341
6. Tofisopamum
7. Uzc80hau42
8. Seriel
9. 5h-2,3-benzodiazepine, 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-
10. 1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-benzo[d][1,2]diazepine
11. Tofizopam
12. Ncgc00165912-02
13. Benzodiazepine; Egyt 341; Grandaxin; Nodeprine; Seriel
14. Tofisopamum [inn-latin]
15. Tofisopam [inn:dcf:jan]
16. 82059-50-5
17. Einecs 244-922-3
18. Unii-uzc80hau42
19. Ccris 8738
20. Emandaxin (tn)
21. 7,8-dimethoxy-1-(3,4-dimethoxyphenyl)-5-ethyl-4-methyl-5h-2,3-benzodiazepine
22. Tofisopam [inn]
23. Tofisopam [jan]
24. Tofisopam [mi]
25. Dsstox_cid_3681
26. Tofisopam (jp17/inn)
27. Tofisopam [mart.]
28. Tofisopam [who-dd]
29. Dsstox_rid_77145
30. Dsstox_gsid_23681
31. Schembl43522
32. Tofisopam (patented In Japan)
33. Chembl404216
34. Schembl8086894
35. Dtxsid3023681
36. Chebi:32241
37. Bcp09600
38. Hy-a0165
39. Tox21_112269
40. Tofisopam, >=98% (hplc), Solid
41. Akos025401672
42. Db08811
43. Sb17495
44. Sb17496
45. Ncgc00165912-01
46. Ncgc00165912-03
47. Ac-24288
48. Ls-14948
49. Cas-22345-47-7
50. Db-045872
51. Ft-0638212
52. T3509
53. D01254
54. 345t477
55. A878592
56. Q945537
57. Q-201839
58. Brd-a69095630-001-01-1
59. 1-(3,4-dimethoxyphenyl)-4-methyl-5-ethyl-7,8-dimethoxy-5h-2,3-benzodiazepine
60. 1-[3,4-bis(methyloxy)phenyl]-5-ethyl-4-methyl-7,8-bis(methyloxy)-5h-2,3-benzodiazepine
61. (+/-)-1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-2,3-benzodiazepine
62. (1z,3z)-1-(3,4-dimethoxyphenyl)-5-ethyl-7,8-dimethoxy-4-methyl-5h-benzo[d][1,2]diazepine
| Molecular Weight | 382.5 g/mol |
|---|---|
| Molecular Formula | C22H26N2O4 |
| XLogP3 | 3.2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 6 |
| Exact Mass | 382.18925731 g/mol |
| Monoisotopic Mass | 382.18925731 g/mol |
| Topological Polar Surface Area | 61.6 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
For the treatment of anxiety and alcohol withdrawal.
Like other benzodiazepines, tofisopam possesses anxiolytic properties but unlike other benzodiazepines it does not have anticonvulsant, sedative, skeletal muscle relaxant, motor skill-impairing or amnestic properties. It enhances the anticonvulsant activity of 1,4-benzodiazepines like diazepam but not sodium valproate, carbamazepine, phenobarbital, or phenytoin.
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA23 - Tofisopam
Hepatic.
6-8 hours
Tofisopam does not bind to the benzodiazepine binding site of the gamma-aminobutyric acid receptor. One study (Rundfeldt C. et al.) has shown that tofisopam acts as an isoenzyme-selective inhibitor of phosphodiesterases (PDEs) with highest affinity to PDE-4A1 (0.42 M) followed by PDE-10A1 (0.92 M), PDE-3 (1.98 M) and PDE-2A3 (2.11 M).


