



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
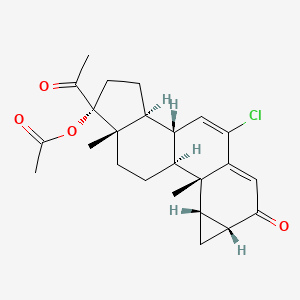

1. Androcur
2. Cyproterone Acetate, (1 Alpha,2 Alpha)-isomer
3. Cyproterone Acetate, (1 Alpha,2 Alpha,9 Beta,10 Alpha)-isomer
4. Cyproterone Acetate, (17 Alpha)-isomer
1. 427-51-0
2. Androcur
3. Cyproterone 17-o-acetate
4. Cyproteroneacetate
5. Cyproteron Acetate
6. Cyproteron-r Acetate
7. Cyprosterone Acetate
8. Sh 714
9. Cyprostat
10. Nsc-81430
11. Sh-714
12. Sh 80714
13. 4km2bn5jhf
14. Cyproterone 17.alpha.-acetate
15. Mls000859908
16. Chembl139835
17. (1r,3as,3br,7ar,8as,8bs,8cs,10as)-1-acetyl-5-chloro-8b,10a-dimethyl-7-oxo-1,2,3,3a,3b,7,7a,8,8a,8b,8c,9,10,10a-tetradecahydrocyclopenta[a]cyclopropa[g]phenanthren-1-yl Acetate
18. 3'h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1beta,2beta)-
19. Chebi:50743
20. Nsc81430
21. 6-chloro-1beta,2beta-dihydro-17-hydroxy-3'h-cyclopropa(1,2)-pregna-1,4,6-triene-3,20-dione Acetate
22. Ncgc00091032-03
23. Dsstox_cid_366
24. Dsstox_rid_75542
25. Dsstox_gsid_20366
26. 3'h-cyclopropa[1,2]pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1.beta.,2.beta.)-
27. 6-chloro-3,20-dioxo-1beta,2beta-dihydro-3'h-cyclopropa[1,2]pregna-4,6-dien-17-yl Acetate
28. (2ar,3as,3bs,3cs,5as,6r,8as,8br)-6-acetyl-10-chloro-3b,5a-dimethyl-2-oxo-2,2a,3,3a,3b,3c,4,5,5a,6,7,8,8a,8b-tetradecahydrocyclopenta[a]cyclopropa[g]phenanthren-6-yl Acetate
29. Smr000326769
30. Ccris 4385
31. Hsdb 3592
32. Sr-01000075755
33. Einecs 207-048-3
34. Unii-4km2bn5jhf
35. Nsc 81430
36. Cyprostat®
37. Cyproterone-acetate
38. (1?,2?)-17-(acetyloxy)-6-chloro-1,2-dihydro-3'h-cyclopropa[1,2]pregna-1,4,6-triene-3,20-dione
39. 3'h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1.beta.,2.beta.)-
40. Cas-427-51-0
41. Mfcd00864671
42. 2oz7
43. 6-chloro-delta-6-1,2alpha-methylene-17alpha-hydroxyprogesterone Acetate
44. 1,2-alpha-methylene-6-chloro-delta(sup 6)-17-alpha-hydroxyprogesterone Acetate
45. 1,2-alpha-methylene-6-chloro-pregna-4,6-diene-3,20-dione 17-alpha-acetate
46. 6-chloro-1,2-alpha-methylene-6-dehydro-17-alpha-hydroxyprogesterone Acetate
47. 6-chloro-delta(sup 6)-1,2-alpha-methylene-17-alpha-hydroxyprogesterone Acetate
48. Cid_9880
49. Schembl5936
50. 1,2-alpha-methylene-6-chloro-(sup 4,6)-pregnadiene-17-alpha-ol-3,20-dione 17-alpha-acetate
51. 17-alpha-acetoxy-6-chloro-1-alpha,2-alpha-methylenepregna-4,6-diene-3,20-dione
52. 6-chlor-delta(sup 6)-1,2-alpha-methylen-17-alpha-hydroxyprogesteronacetat [german]
53. 6-chloro-1,2-alpha-methylene-17-alpha-hydroxy-delta(sup 6)-progesterone Acetate
54. 6-chloro-17-hydroxy-1-alpha,2-alpha-methylenepregna-4,6-diene-3,20-dione Acetate
55. Pregna-4,6-diene-3,20-dione, 6-chloro-17-hydroxy-1-alpha,2-alpha-methylene-, Acetate
56. Bidd:pxr0052
57. Lopac0_000301
58. Mls001055462
59. Mls001066353
60. Mls002207305
61. Mls006011110
62. Cyproterone Acetate, >=98%
63. (non-d)cyproterone Acetate-d5
64. Gtpl2865
65. Cyproterone Acetate [mi]
66. Dtxsid5020366
67. Cyproterone Acetate [jan]
68. Cyproterone Acetate Assay Standard
69. Cyproterone Acetate [hsdb]
70. Cyproterone Acetate [usan]
71. Hms2090a14
72. Hms2233j06
73. Hms3260n04
74. Cyproterone Acetate [mart.]
75. Zinc3814423
76. Tox21_111064
77. Tox21_201686
78. Tox21_302941
79. Tox21_500301
80. Ac-929
81. Bdbm50094569
82. Cyproterone Acetate [who-dd]
83. Dl-368
84. S2042
85. 6-chlor-delta(sup 6)-1,2-alpha-methylen-17-alpha-hydroxyprogesteronacetat
86. Akos008901350
87. Akos015895238
88. Tox21_111064_1
89. Ccg-204396
90. Db04839
91. Lp00301
92. Sdccgsbi-0050289.p002
93. Ncgc00091032-01
94. Ncgc00091032-04
95. Ncgc00091032-05
96. Ncgc00091032-09
97. Ncgc00091032-12
98. Ncgc00256442-01
99. Ncgc00259235-01
100. Ncgc00260986-01
101. Ncgc00262575-02
102. Progesterone,2.alpha.-methylene, Acetate
103. (1s,2s,3s,12s,16s,5r,11r,15r)-15-acetyl-9-chloro-2,16-dimethyl-6-oxopentacyclo [9.7.0.0<2,8>.0<3,5>.0<12,16>]octadeca-7,9-dien-15-yl Acetate
104. 3'h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione, 6-chloro-1-beta,2-beta-dihydro-17-hydroxy-, Acetate
105. 6-chloro-1-beta,2-beta-dihydro-17-hydroxy-3'h-cyclopropa(1,2)pregna-1,4,6-triene-3,20-dione 17-acetate
106. Cyproterone Acetate [ep Monograph]
107. Smr000686068
108. Cyproterone Acetate For Peak Identification
109. Progesterone,2.alpha.-methylene-, Acetate
110. (acetyl-chloro-dimethyl-oxo-[?]yl) Acetate
111. 1,6-diene-3,20-dione 17.alpha.-acetate
112. Eu-0100301
113. C 3412
114. D01368
115. Ab00698312-08
116. 1,6)-pregnadeine-17.alpha.-3,20-dione Acetate
117. 427c510
118. Q426185
119. Sr-01000075755-1
120. Sr-01000075755-4
121. Sr-01000075755-6
122. W-106262
123. 1,6)-pregnadiene-17.alpha.-ol-3,20-dione Acetate
124. Brd-k41141507-001-16-2
125. Wln: L F3 E6 D665 Iv Ju Lutj A1 E1 Rv1 Rq
126. 17.alpha.-acetoxy-6-chloro-1.alpha.,6-diene-3,20-dione
127. 6-chloro-17-hydroxy-1.alpha.,6-diene-3,20-dione Acetate
128. 1,6)-pregnadiene-17.alpha.-ol-3,20-dione 17alpha-acetate
129. 6-chloro-1.beta.,2]pregna-1,4,6-triene-3,20-dione Acetate
130. Cyproterone Acetate, European Pharmacopoeia (ep) Reference Standard
131. 6-chloro-17-acetoxy-1alpha,2alpha-methylene-4,6-pregnadiene-3,20-dione
132. 1,2.alpha.-methylene-6-chloro-.delta.(sup 6)-17.alpha.-hydroxyprogesterone Acetate
133. 17alpha-acetoxy-6-chloro-1alpha,2alpha-methylene-4,6-pregnadiene-3,20-dione
134. 6-chloro-.delta.(sup 6)-1,2.alpha.-methylene-17.alpha.-hydroxyprogesterone Acetate
135. 6-chloro-1,2.alpha.-methylene-17.alpha.-hydroxy-.delta.(sup 6)-progesterone Acetate
136. 6-chloro-1,2.alpha.-methylene-6-dehydro-17.alpha.-hydroxyprogesterone Acetate
137. Cyproterone Acetate For Peak Identification, European Pharmacopoeia (ep) Reference Standard
138. Pregna-4,20-dione, 6-chloro-17-hydroxy-1.alpha.,2.alpha.-methylene-, Acetate
139. 3'h-cyclopropa[1,4,6-triene-3,20-dione, 17-(acetyloxy)-6-chloro-1,2-dihydro-, (1.beta.,2.beta.)-
140. 3'h-cyclopropa[1,4,6-triene-3,20-dione, 6-chloro-1.beta.,2.beta.-dihydro-17-hydroxy-, Acetate
141. 3'h-cyclopropa[1,4,6-triene-3,20-dione, 6-chloro-1.beta.,2.beta.-dihydro-17-hydroxy-,acetate
142. 6-chloro-1.beta.,2.beta.-dihydro-17-hydroxy-3'h-cyclopropa(1,2)-pregna-1,4,6-triene-3,20-dione Acetate
143. 6-chloro-3,20-dioxo-1beta,2beta-dihydro-3''h-cyclopropa[1,2]pregna-4,6-dien-17-yl Acetate
144. Acetic Acid (8r,9s,10s,13s,14s,17r)-17-acetyl-6-(s)-chloro-10,13-dimethyl-3-(r)-oxo-1,2,3,8,9,10,11,12,13,14,15,16,17,20-tetradecahydro-cyclopropa[1,2]cyclopenta[a]phenanthren-17-yl Ester
| Molecular Weight | 416.9 g/mol |
|---|---|
| Molecular Formula | C24H29ClO4 |
| XLogP3 | 3.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 416.1754371 g/mol |
| Monoisotopic Mass | 416.1754371 g/mol |
| Topological Polar Surface Area | 60.4 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 903 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 8 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Androgen Antagonists; Antineoplastic Agents; Contraceptive Agents, Male; Progestational Hormones, Synthetic
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/Cyproterone is indicated for the/ control of libido in severe hypersexuality and/or sexual deviation in the adult male.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
/Cyproterone is indicated for the/ management of patients with prostatic cancer (1) to suppress "flare" with initial LHRH analogue therapy,(2) in long-term palliative treatment where LHRH analogues or surgery are contraindicated, not tolerated, or where oral therapy is preferred, and (3) in the treatment of hot flushes in patients under treatment with LHRH analogues or who have had orchidectomy.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
Dianette (cyproterone acetate/ethinylestradiol) is recommended for use in women only for the treatment of (a) severe acne, refractory to prolonged oral antibiotic therapy; (b) moderately severe hirsutism.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Dianette (Last updated November 2010). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1814/SPC/Dianette/
Although Dianette also acts as an oral contraceptive, it should not be used in women solely for contraception, but should be reserved for those women requiring treatment for the androgen-dependent conditions.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Dianette (Last updated November 2010). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1814/SPC/Dianette/
Direct hepatic toxicity, including jaundice, hepatitis and hepatic failure, has been observed in patients treated with Cyprostat. At dosages of 100 mg and above cases with fatal outcome have also been reported. Most reported fatal cases were in men with advanced prostatic cancer. Toxicity is dose-related and develops, usually, several months after treatment has begun. Liver function tests should be performed pre-treatment, regularly during treatment and whenever any symptoms or signs suggestive of hepatotoxicity occur. If hepatotoxicity is confirmed, Cyprostat should be withdrawn, unless the hepatotoxicity can be explained by another cause, eg metastatic disease, in which case Cyprostat should be continued only if the perceived benefit outweighs the risk.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
In very rare cases benign and malignant liver tumors, which may lead to life-threatening intra-abdominal hemorrhage, have been observed after the use of Cyprostat. If severe upper abdominal complaints, liver enlargement or signs of intra-abdominal hemorrhage occur, a liver tumor should be considered in the differential diagnosis.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
The occurrence of thromboembolic events has been reported in patients using Cyprostat, although a causal relationship has not been established. Patients with previous arterial or venous thrombotic/thromboembolic events (eg deep vein thrombosis, pulmonary embolism, myocardial infarction), with a history of cerebrovascular accidents or with advanced malignancies are at increased risk of further thromboembolic events, and may be at risk of recurrence of the disease during Cyprostat therapy.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
In patients with a history of thromboembolic processes or suffering from sickle-cell anemia or severe diabetes with vascular changes, the risk: benefit ratio must be considered carefully in each individual case before Cyprostat is prescribed.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
For more Drug Warnings (Complete) data for CYPROTERONE ACETATE (17 total), please visit the HSDB record page.
For the palliative treatment of patients with advanced prostatic carcinoma.
Cyproterone is an antiandrogen. It suppresses the actions of testosterone (and its metabolite dihydrotestosterone) on tissues. It acts by blocking androgen receptors which prevents androgens from binding to them and suppresses luteinizing hormone (which in turn reduces testosterone levels).
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Androgen Antagonists
Compounds which inhibit or antagonize the biosynthesis or actions of androgens. (See all compounds classified as Androgen Antagonists.)
Contraceptive Agents, Male
Chemical substances or agents with contraceptive activity in males. Use for male contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Male.)
Absorption
Completely absorbed following oral administration.
Route of Elimination
It is excreted approximately 60% in the bile and 33% through the kidneys.
Following oral administration, cyproterone acetate is completely absorbed over a wide dose range. The ingestion of two cyproterone acetate 50 mg tablets gives maximum serum levels of about 285 ng/mL at about 3 hours. Thereafter, drug serum levels declined during a time interval of typically 24 to 120 hr, with a terminal half-life of 43.9 +/- 12.8 hr. The total clearance of cyproterone acetate from serum is 3.5 +/- 1.5 mL/min/kg.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
The absolute bioavailability of cyproterone acetate is almost complete (88% of dose).
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
Some drug is excreted unchanged with bile fluid. Most of the dose is excreted in the form of metabolites at a urinary to biliary ratio of 3:7.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
Cyproterone acetate is almost exclusively bound to plasma albumin. About 3.5 - 4% of total drug levels are present unbound. Because protein binding is non-specific, changes in SHBG (sex hormone binding globulin) levels do not affect the pharmacokinetics of cyproterone acetate.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
For more Absorption, Distribution and Excretion (Complete) data for CYPROTERONE ACETATE (6 total), please visit the HSDB record page.
Primarily hepatic. Cyproterone acetate is metabolized by the CYP3A4 enzyme, forming the active metabolite 15beta-hydroxycyproterone acetate, which retains its antiandrogen activity, but has reduced progestational activity.
Cyproterone acetate is metabolized by various pathways, including hydroxylations and conjugations. The main metabolite in human plasma is the 15beta-hydroxy derivative.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
Elimination Following oral or intramuscular administration, the plasma half-life is 38 and 96 hours, respectively.
The renal and biliary excretion proceeds with a half-life of 1.9 days. Metabolites from plasma are eliminated at a similar rate (half-life of 1.7 days).
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
Terminal half-life of 43.9 +/- 12.8 hr.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Androcur (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1808/SPC/Androcur/
The direct antiandrogenic effect of cyproterone is blockage of the binding of dihydrotestosterone to the specific receptors in the prostatic carcinoma cell. In addition, cyproterone exerts a negative feed-back on the hypothalamo-pituitary axis, by inhibiting the secretion of luteinizing hormone resulting in diminished production of testicular testosterone.
Prostatic carcinoma and its metastases are in general androgen-dependent. Cyproterone acetate exerts a direct anti-androgen action on the tumor and its metastases. It also has progestogenic activity, which exerts a negative feedback effect on the hypothalamic receptors, so leading to a reduction in gonadotrophin release, and hence to diminished production of testicular androgens. Sexual drive and potency are reduced and gonadal function is inhibited.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
Cell proliferation and cell death appear in several systems as mutually exclusive, which raises the assumption that a same factor or secondary signal(s) might exert opposite control on the two processes. To test this assumption we investigated the time-course evolution of the S phase and apoptotic indices in rat liver during cyproterone acetate (CPA) induced hyperplasia and during the recovery of normal liver mass provoked, respectively, by cyproterone acetate (CPA) treatment and withdrawal. The levels of c-myc and c-ras transcripts were also followed in view of the indications of a positive role of these oncogenes in proliferation. The data showed that proliferation and cell death are not always mutually exclusive and that a high rate of cell death was indifferently associated with high or low c-ras expression. Our data are consistent with a role of this gene in proliferation but exclude that it plays an opposite role in controlling cell death.
PMID:1388096 Servais P, Galand P; Cell Biol Int Rep 16 (4): 319-28 (1992)
The antigonadotropic effect of cyproterone acetate is also exerted when administered with LHRH analogues. The initial increase of testosterone caused by this class of substances is reduced by cyproterone acetate. An occasional tendency for the prolactin levels to increase slightly has been observed under higher doses of cyproterone acetate.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Cyprostat (Last updated January 2011). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/20815/SPC/Cyprostat+100mg/
Dianette blocks androgen-receptors. It also reduces androgen synthesis both by negative feedback effect on the hypothalamo-pituitiary-ovarian systems and by the inhibition of androgen-synthesising enzymes.
Datapharm Communications Ltd; Electronic Medicines Compendium (eMC), Summary of Product Characteristics (SPC) for Dianette (Last updated November 2010). Available from, as of March 23, 2011: https://www.medicines.org.uk/EMC/medicine/1814/SPC/Dianette/
For more Mechanism of Action (Complete) data for CYPROTERONE ACETATE (8 total), please visit the HSDB record page.


