



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
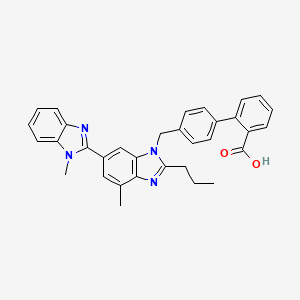

1. 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1h-benzimidazol)-1'-yl)methyl)-(1,1'-biphenyl)-2-carboxylic Acid
2. Bibr 277
3. Bibr-277
4. Micardis
5. Pritor
1. 144701-48-4
2. Micardis
3. Pritor
4. Kinzalmono
5. Bibr 277
6. Semintra
7. 4'-((1,7'-dimethyl-2'-propyl-1h,3'h-[2,5'-bibenzo[d]imidazol]-3'-yl)methyl)-[1,1'-biphenyl]-2-carboxylic Acid
8. Bibr-277
9. Tolura
10. Bibr 277se
11. Telmisartan [inn]
12. Telmisattan
13. Telmisartan Teva
14. Telmisartan Actavis
15. Bibr-277-se
16. 4'-[(1,7'-dimethyl-2'-propyl-1h,3'h-2,5'-bibenzimidazol-3'-yl)methyl]biphenyl-2-carboxylic Acid
17. Bibr-277se
18. Bibr 277 Se
19. C09ca07
20. 2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propylbenzimidazol-1-yl]methyl]phenyl]benzoic Acid
21. Mfcd00918125
22. 4'-((4-methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl)methyl)-2-biphenylcarboxylic Acid
23. Chembl1017
24. U5syw473rq
25. Bibr-277 Se
26. Chebi:9434
27. 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1h-benzimidazol)-1'-yl)methyl)-(1,1'-biphenyl)-2-carboxylic Acid
28. (1,1'-biphenyl)-2-carboxylic Acid, 4'-((1,4'-dimethyl-2'-propyl(2,6'-bi-1h-benzimidazol)-1'-yl)methyl)-
29. [1,1'-biphenyl]-2-carboxylic Acid, 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1h-benzimidazol]-1'-yl)methyl]-
30. Ncgc00095150-01
31. Targit
32. Dsstox_cid_3636
33. Dsstox_rid_77121
34. Dsstox_gsid_23636
35. 2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propyl-benzimidazol-1-yl]methyl]phenyl]benzoic Acid
36. 4'-[(1,4'-dimethyl-2'propyl[2,6'-bi-1h-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic Acid
37. 4'[(1,4'-dimethyl-2'-propyl[2,6'-bi-1h-benzimidazol]-1'-yl)methyl][1,1'-biphenyl]-2-carboxylic Acid
38. Kinzal
39. Tazlok
40. Kinzal/pritor
41. Micardis (tn)
42. 4'-((1,7'-dimethyl-2'-propyl-1h,3'h-2,5'-bibenzo[d]imidazol-3'-yl)methyl)biphenyl-2-carboxylic Acid
43. Smr000466326
44. Cas-144701-48-4
45. Bay 68-9291
46. Sr-01000759355
47. Unii-u5syw473rq
48. Telmisartana
49. Telmisartar
50. Telsite
51. Telday
52. Hsdb 7590
53. 4'-[(1,4'-dimethyl-2'-propyl[2,6'-bi-1h-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic Acid
54. 4'-[[4-methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl]methyl]-2-biphenylcarboxylic Acid
55. Telmisartan [usan:usp:inn:ban]
56. Telmisartan- Bio-x
57. Ym-086
58. Bay68-9291
59. Telmisartan (micardis)
60. Cpd000466326
61. Telmisartan [mi]
62. Spectrum2_001976
63. Spectrum3_001089
64. Spectrum4_001261
65. Spectrum5_001053
66. Telmisartan [jan]
67. Telmisartan [hsdb]
68. Telmisartan [usan]
69. Telmisartan [vandf]
70. Schembl4464
71. Telmisartan [mart.]
72. Bspbio_002738
73. Gtpl592
74. Kbiogr_001842
75. Telmisartan [usp-rs]
76. Telmisartan [who-dd]
77. Mls000759432
78. Mls001076687
79. Mls001424174
80. Mls006011851
81. Bidd:gt0365
82. Spectrum1505261
83. Telmisartan Teva Pharma
84. Spbio_002131
85. Telmisartan [ema Epar]
86. Telmisartan (jp17/usp/inn)
87. Dtxsid8023636
88. Telmisartan [green Book]
89. Kbio3_001958
90. Telmisartan [ep Impurity]
91. Telmisartan [orange Book]
92. Telmisartan For System Suitability
93. Hms1922p07
94. Hms2051p16
95. Hms2090p17
96. Hms2093m22
97. Hms2231p07
98. Hms3393p16
99. Hms3655c08
100. Hms3715l17
101. Hms3750e19
102. Pharmakon1600-01505261
103. Telmisartan [ep Monograph]
104. Telmisartan For Peak Identification
105. Telmisartan [usp Monograph]
106. Albb-028954
107. Bcp04513
108. Onduarp Component Telmisartan
109. Twynsta Component Telmisartan
110. Zinc1530886
111. Tox21_111452
112. Bbl029085
113. Bdbm50043280
114. Ccg-39514
115. Dl-511
116. Nsc759811
117. S1738
118. Stk624049
119. Tolucombi Component Telmisartan
120. Akos005557501
121. Kinzalkomb Component Telmisartan
122. Pritorplus Component Telmisartan
123. Telmisartan, >=98% (hplc), Solid
124. Tox21_111452_1
125. Ab07687
126. Ac-2013
127. Am90292
128. Cs-1699
129. Db00966
130. Ks-1215
131. Nc00296
132. Nsc 759811
133. Nsc-759811
134. Telmisartan Component Of Onduarp
135. Telmisartan Component Of Twynsta
136. Bay-68-9291
137. Micardis Hct Component Telmisartan
138. Micardisplus Component Telmisartan
139. Ncgc00095150-02
140. Ncgc00095150-03
141. Ncgc00095150-04
142. Ncgc00095150-06
143. Ncgc00095150-07
144. Ncgc00095150-08
145. Telmisartan [ema Epar Veterinary]
146. Telmisartan Component Of Tolucombi
147. 2-(4-{[4-methyl-6-(1-methyl-1h-1,3-benzodiazol-2-yl)-2-propyl-1h-1,3-benzodiazol-1-yl]methyl}phenyl)benzoic Acid
148. 4'-[(1,7'-dimethyl-2'-propyl-1h,3'h-2,5'-bibenzimidazol-3'-yl)methyl][1,1'-biphenyl]-2-carboxylic Acid
149. Bt164444
150. Hy-13955
151. Sy052776
152. Telmisartan Component Of Kinzalkomb
153. Telmisartan Component Of Pritorplus
154. Bcp0726000055
155. Sbi-0206733.p001
156. Telmisartan Component Of Micardiplus
157. Telmisartan Component Of Micardisplus
158. Ft-0631170
159. Ft-0674836
160. Sw197676-3
161. T-170
162. T2861
163. C07710
164. D00627
165. Ab00639941-06
166. Ab00639941-07
167. Ab00639941_08
168. Ab00639941_09
169. 701t484
170. A808270
171. L001035
172. Q733186
173. Q-101933
174. Sr-01000759355-4
175. Sr-01000759355-5
176. Brd-k73999723-001-02-2
177. Z2210710360
178. Telmisartan, European Pharmacopoeia (ep) Reference Standard
179. Telmisartan, United States Pharmacopeia (usp) Reference Standard
180. Telmisartan, Pharmaceutical Secondary Standard; Certified Reference Material
181. 4-(1,4-dimethyl-2-propyl-2,6-bi-1h-benzimidazol-1-yl)methyl-1,1-biphenyl-2-carboxylicacid
182. Telmisartan For Peak Identification, European Pharmacopoeia (ep) Reference Standard
183. Telmisartan For System Suitability, European Pharmacopoeia (ep) Reference Standard
184. 2-[4-[[4-methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl]methyl]phenyl]benzoic Acid
185. 2-[4-[[4-methyl-6-(1-methylbenzimidazol-2-yl)-2-propylbenzimidazol-1-yl]methyl]phenyl]benzoic Acid.
186. 4''-((1,4''-dimethyl-2''-propyl(2,6''-bi-1h-benzimidazol)-1''-yl)methyl)-(1,1''-biphenyl)-2-carboxylic Acid
187. 4''-((4-methyl-6-(1-methyl-2-benzimidazolyl)-2-propyl-1-benzimidazolyl)methyl)-2-biphenylcarboxylic Acid
188. 4''-[(1,4''-dimethyl-2''propyl[2,6''-bi-1h-benzimidazol]-1''-yl)methyl]-[1,1''-biphenyl]-2-carboxylic Acid
189. 4''-[(1,7''-dimethyl-2''-propyl-1h,3''h-2,5''-bibenzimidazol-3''-yl)methyl][1,1''-biphenyl]-2-carboxylic Acid
190. 4''-[(1,7''-dimethyl-2''-propyl-1h,3''h-2,5''-bibenzimidazol-3''-yl)methyl]biphenyl-2-carboxylic Acid
191. 4'-((1,7'-dimethyl-2'-propyl-1h,3'h-[2,5'-bibenzo[d]imidazol]-3'-yl)methyl)-[1,1'-biphenyl]-2-carboxylicacid
192. 4'-(1,7'-dimethyl-2'-propyl-1h-[2,5']bibenzoimidazolyl-3'-ylmethyl)-biphenyl-2-carboxylic Acid
193. 4'-[[2-n-propyl-4-methyl-6-(1-methylbenzimidazol-2-yl)-benzimidazol-1-yl]-methyl]-biphenyl-2-carboxylic Acid
194. 4'-[[4-methyl-6-(1-methyl-1h-benzimidazol-2-yl)-2-propyl-1h-benzimidazol-1-yl]methyl]biphenyl-2-carboxylic Acid
195. 4'-[2-n-propyl-4-methyl-6-(1-methyl Benzimidazol-2-yl)benzimidazol-1-yl Methyl]biphenyl-2-carboxylic Acid
196. 4'-{[4-methyl-6-(1-methyl-1h-1,3-benzodiazol-2-yl)-2-propyl-1h-1,3-benzodiazol-1-yl]methyl}-[1,1'-biphenyl]-2-carboxylic Acid
197. Tls
| Molecular Weight | 514.6 g/mol |
|---|---|
| Molecular Formula | C33H30N4O2 |
| XLogP3 | 6.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 7 |
| Exact Mass | 514.23687621 g/mol |
| Monoisotopic Mass | 514.23687621 g/mol |
| Topological Polar Surface Area | 72.9 Ų |
| Heavy Atom Count | 39 |
| Formal Charge | 0 |
| Complexity | 831 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 4 | |
|---|---|
| Drug Name | Micardis |
| PubMed Health | Telmisartan (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | MICARDIS is a non-peptide angiotensin II receptor (type AT1) antagonist.Telmisartan is chemically described as 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empiri |
| Active Ingredient | Telmisartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 2 of 4 | |
|---|---|
| Drug Name | Telmisartan |
| PubMed Health | Telmisartan (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Telmisartanis a non-peptide angiotensin II receptor (type AT1) antagonist. Telmisartan is chemically described as 4-[(1,4-dimethyl-2-propyl [2,6-bi-1H-benzimidazol]-1-yl)methyl]-[1,1-biphenyl]-2-carboxylic acid. Its empirical formul... |
| Active Ingredient | Telmisartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Alembic Pharms; Torrent Pharms; Watson Labs; Glenmark Generics; Zydus Pharms Usa |
| 3 of 4 | |
|---|---|
| Drug Name | Micardis |
| PubMed Health | Telmisartan (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | MICARDIS is a non-peptide angiotensin II receptor (type AT1) antagonist.Telmisartan is chemically described as 4'-[(1,4'-dimethyl-2'-propyl [2,6'-bi-1H-benzimidazol]-1'-yl)methyl]-[1,1'-biphenyl]-2-carboxylic acid. Its empiri |
| Active Ingredient | Telmisartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 4 of 4 | |
|---|---|
| Drug Name | Telmisartan |
| PubMed Health | Telmisartan (By mouth) |
| Drug Classes | Antihypertensive, Cardiovascular Agent, Renal Protective Agent |
| Drug Label | Telmisartanis a non-peptide angiotensin II receptor (type AT1) antagonist. Telmisartan is chemically described as 4-[(1,4-dimethyl-2-propyl [2,6-bi-1H-benzimidazol]-1-yl)methyl]-[1,1-biphenyl]-2-carboxylic acid. Its empirical formul... |
| Active Ingredient | Telmisartan |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 80mg; 40mg; 20mg |
| Market Status | Prescription |
| Company | Mylan Pharms; Alembic Pharms; Torrent Pharms; Watson Labs; Glenmark Generics; Zydus Pharms Usa |
Angiotensin II Type 1 Receptor Blockers; Antihypertensive Agents
National Library of Medicine's Medical Subject Headings. Telmisartan. Online file (MeSH, 2014). Available from, as of September 2, 2014: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
Micardis is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes including the class to which this drug principally belongs. Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than one drug to achieve blood pressure goals. ... Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly. Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal. Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy. /Micardis/ may be used alone or in combination with other antihypertensive agents /Included in US product labeling/
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Micardis is indicated for reduction of the risk of myocardial infarction, stroke, or death from cardiovascular causes in patients 55 years of age or older at high risk of developing major cardiovascular events who are unable to take ACE inhibitors. High risk for cardiovascular events can be evidenced by a history of coronary artery disease, peripheral arterial disease, stroke, transient ischemic attack, or high-risk diabetes (insulin-dependent or non-insulin dependent) with evidence of end-organ damage. Micardis can be used in addition to other needed treatment (such as antihypertensive, antiplatelet or lipid-lowering therapy). Studies of telmisartan in this setting do not exclude the possibility that telmisartan may not preserve a meaningful fraction of the effect of the ACE inhibitor to which it was compared. Consider using the ACE inhibitor first, and, if it is stopped for cough only, consider re-trying the ACE inhibitor after the cough resolves. /Included in US product label/
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Both angiotensin II receptor antagonists /including telmisartan/ and ACE inhibitors have been shown to slow the rate of progression of renal disease in hypertensive patients with diabetes mellitus and microalbuminuria or overt nephropathy, and use of a drug from either class is recommended in such patients. /NOT included in US product label/
American Society of Health-System Pharmacists 2014; Drug Information 2014. Bethesda, MD. 2014, p. 2072
For more Therapeutic Uses (Complete) data for TELMISARTAN (8 total), please visit the HSDB record page.
/BOXED WARNING/ WARNING: FETAL TOXICITY. When pregnancy is detected, discontinue Micardis as soon as possible. Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue Micardis as soon as possible. These adverse outcomes are usually associated with use of these drugs in the second and third trimester of pregnancy. Most epidemiologic studies examining fetal abnormalities after exposure to antihypertensive use in the first trimester have not distinguished drugs affecting the renin-angiotensin system from other antihypertensive agents. Appropriate management of maternal hypertension during pregnancy is important to optimize outcomes for both mother and fetus. In the unusual case that there is no appropriate alternative to therapy with drugs affecting the reninangiotensin system for a particular patient, apprise the mother of the potential risk to the fetus. Perform serial ultrasound examinations to assess the intra-amniotic environment. If oligohydramnios is observed, discontinue Micardis, unless it is considered lifesaving for the mother. Fetal testing may be appropriate, based on the week of pregnancy. Patients and physicians should be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Neonates with a history of in utero exposure to Micardis: If oliguria or hypotension occurs, direct attention toward support of blood pressure and renal perfusion. Exchange transfusions or dialysis may be required as a means of reversing hypotension and/or substituting for disordered renal function.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
FDA Pregnancy Risk Category: D /POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective./
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
For more Drug Warnings (Complete) data for TELMISARTAN (17 total), please visit the HSDB record page.
Used alone or in combination with other classes of antihypertensives for the treatment of hypertension. Also used in the treatment of diabetic nephropathy in hypertensive patients with type 2 diabetes mellitus, as well as the treatment of congestive heart failure (only in patients who cannot tolerate ACE inhibitors).
FDA Label
Treatment of essential hypertension in adults.
Reduction of proteinuria associated with chronic kidney disease (CKD).
* Hypertension:
Treatment of essential hypertension in adults.
* Cardiovascular prevention:
Reduction of cardiovascular morbidity in patients with:
- manifest atherothrombotic cardiovascular disease (history of coronary heart disease or peripheral arterial disease) or;
- type 2 diabetes mellitus with documented target organ damage.
* Hypertension:
Treatment of essential hypertension in adults.
* Cardiovascular prevention:
Reduction of cardiovascular morbidity in patients with:
- manifest atherothrombotic cardiovascular disease (history of coronary heart disease, stroke, or peripheral arterial disease) or;
- type-2 diabetes mellitus with documented target-organ damage.
Treatment of essential hypertension in adults
* Hypertension:
Treatment of essential hypertension in adults.
* Cardiovascular prevention:
Reduction of cardiovascular morbidity in patients with:
- manifest atherothrombotic cardiovascular disease (history of coronary heart disease, stroke, or peripheral arterial disease) or;
- type 2 diabetes mellitus with documented target organ damage.
* Hypertension:
Treatment of essential hypertension in adults.
* Cardiovascular prevention:
Reduction of cardiovascular morbidity in patients with:
- manifest atherothrombotic cardiovascular disease (history of coronary heart disease, stroke, or peripheral arterial disease) or;
- type-2 diabetes mellitus with documented target-organ damage.
* Hypertension:
Treatment of essential hypertension in adults.
* Cardiovascular prevention:
Reduction of cardiovascular morbidity in patients with:
- manifest atherothrombotic cardiovascular disease (history of coronary heart disease, stroke, or peripheral arterial disease) or;
- type-2 diabetes mellitus with documented target-organ damage.
Treatment of essential hypertension in adults:
* Add on therapy:
Onduarp is indicated in adults whose blood pressure is not adequately controlled on amlodipine.
* Replacement therapy:
Adult patients receiving telmisartan and amlodipine from separate tablets can instead receive tablets of Onduarp containing the same component doses.
Telmisartan is an orally active nonpeptide angiotensin II antagonist that acts on the AT1 receptor subtype. It has the highest affinity for the AT1 receptor among commercially available ARBS and has minimal affinity for the AT2 receptor. New studies suggest that telmisartan may also have PPARγ agonistic properties that could potentially confer beneficial metabolic effects, as PPARγ is a nuclear receptor that regulates specific gene transcription, and whose target genes are involved in the regulation of glucose and lipid metabolism, as well as anti-inflammatory responses. This observation is currently being explored in clinical trials. Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Telmisartan works by blocking the vasoconstrictor and aldosterone secretory effects of angiotensin II.
Angiotensin II Type 1 Receptor Blockers
Agents that antagonize ANGIOTENSIN II TYPE 1 RECEPTOR. Included are ANGIOTENSIN II analogs such as SARALASIN and biphenylimidazoles such as LOSARTAN. Some are used as ANTIHYPERTENSIVE AGENTS. (See all compounds classified as Angiotensin II Type 1 Receptor Blockers.)
Antihypertensive Agents
Drugs used in the treatment of acute or chronic vascular HYPERTENSION regardless of pharmacological mechanism. Among the antihypertensive agents are DIURETICS; (especially DIURETICS, THIAZIDE); ADRENERGIC BETA-ANTAGONISTS; ADRENERGIC ALPHA-ANTAGONISTS; ANGIOTENSIN-CONVERTING ENZYME INHIBITORS; CALCIUM CHANNEL BLOCKERS; GANGLIONIC BLOCKERS; and VASODILATOR AGENTS. (See all compounds classified as Antihypertensive Agents.)
C09CA07
QC09CA07
C09CA07
C09CA07
C09CA07
C09CA07
C09CA07
C09CA07
C09DB04
C09CA07
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C09 - Agents acting on the renin-angiotensin system
C09C - Angiotensin ii receptor blockers (arbs), plain
C09CA - Angiotensin ii receptor blockers (arbs), plain
C09CA07 - Telmisartan
Absorption
Absolute bioavailability depends on dosage. Food slightly decreases the bioavailability (a decrease of about 6% is seen when the 40-mg dose is administered with food).
Route of Elimination
Following either intravenous or oral administration of 14C-labeled telmisartan, most of the administered dose (>97%) was eliminated unchanged in feces via biliary excretion; only minute amounts were found in the urine (0.91% and 0.49% of total radioactivity, respectively).
Volume of Distribution
500 L
Clearance
>800 mL/min
Following either intravenous or oral administration of (14)C-labeled telmisartan, most of the administered dose (>97%) was eliminated unchanged in feces via biliary excretion; only minute amounts were found in the urine (0.91% and 0.49% of total radioactivity, respectively).
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Following oral administration, peak concentrations (Cmax) of telmisartan are reached in 0.5 to 1 hour after dosing. Food slightly reduces the bioavailability of telmisartan, with a reduction in the area under the plasma concentration-time curve (AUC) of about 6% with the 40 mg tablet and about 20% after a 160 mg dose. The absolute bioavailability of telmisartan is dose dependent. At 40 and 160 mg the bioavailability was 42% and 58%, respectively. The pharmacokinetics of orally administered telmisartan are nonlinear over the dose range 20 to 160 mg, with greater than proportional increases of plasma concentrations (Cmax and AUC) with increasing doses. Telmisartan shows bi-exponential decay kinetics with a terminal elimination half life of approximately 24 hours. Trough plasma concentrations of telmisartan with once daily dosing are about 10% to 25% of peak plasma concentrations. Telmisartan has an accumulation index in plasma of 1.5 to 2.0 upon repeated once daily dosing.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Telmisartan is highly bound to plasma proteins (>99.5%), mainly albumin and a1 - acid glycoprotein. Plasma protein binding is constant over the concentration range achieved with recommended doses. The volume of distribution for telmisartan is approximately 500 liters indicating additional tissue binding.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
It is not known whether telmisartan is excreted in human milk, but telmisartan was shown to be present in the milk of lactating rats.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
To study the pharmacolkinetics of telmisartan in healthy Chinese male subjects after oral administration of two dosage levels, 36 healthy subjects were divided into two groups and given a single oral dose of 40 or 80 mg telmisartan (CAS 144701-48-4, MicardisPlus). A sensitive liquid chromatography-tandem mass spectrometry method (LC-MS-MS) was used for the determination of telmisartan in plasma. Both, a non-compartmental and compartmental method were used for analysis of parameters of kinetics. The main pharmacokinetic parameters of the 40 mg and 80 mg regimen group were as follows: t(max) (1.76 +/- 1.75) h, (1.56 +/- 1.09) h, C(max) (163.2 +/- 128.4) ng/mL, (905.7 +/- 583.4) ng/mL, t1/2 (23.6 +/- 10.8) h, (23.0 +/- 6.4) h, AUC(o-t) (1456 +/- 1072) ng x h/mL, (6759 +/- 3754) ng x h/mL, AUC(o-infinity (1611 +/- 1180) ng x h/mL, (7588 +/- 4661) ng x h/mL, respectively. After dose normalization, there was significant difference for main pharmacokinetic parameters C(max) AUC(o-t) and AUC(o-infinity) between two dosage level groups. The plasma concentration-time profile of telmisartan was characterized by a high degree of inter-individual variability and the disposition of telmisartan in healthy Chinese subjects was dose-dependent. The pharmacokinetic parameters C(max) and AUC(o-inifinity) of the 80 mg regimen group increased to about 5-fold compared to that of the 40 mg regimen group, but there was no significant difference for t(max) and t1/2 between the two dose groups.
PMID:17009837 Zhang P et al; Arzneimittelforschung 56 (8): 569-73 (2006)
Minimally metabolized by conjugation to form a pharmacologically inactive acylglucuronide; the glucuronide of the parent compound is the only metabolite that has been identified in human plasma and urine. The cytochrome P450 isoenzymes are not involved in the metabolism of telmisartan.
Telmisartan is metabolized by conjugation to form a pharmacologically inactive acyl glucuronide; the glucuronide of the parent compound is the only metabolite that has been identified in human plasma and urine. After a single dose, the glucuronide represents approximately 11% of the measured radioactivity in plasma. The cytochrome P450 isoenzymes are not involved in the metabolism of telmisartan.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Bi-exponential decay kinetics with a terminal elimination half-life of approximately 24 hours.
Telmisartan shows bi-exponential decay kinetics with a terminal elimination half life of approximately 24 hours.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
Telmisartan interferes with the binding of angiotensin II to the angiotensin II AT1-receptor by binding reversibly and selectively to the receptors in vascular smooth muscle and the adrenal gland. As angiotensin II is a vasoconstrictor, which also stimulates the synthesis and release of aldosterone, blockage of its effects results in decreases in systemic vascular resistance. Telmisartan does not inhibit the angiotensin converting enzyme, other hormone receptors, or ion channels. Studies also suggest that telmisartan is a partial agonist of PPARγ, which is an established target for antidiabetic drugs. This suggests that telmisartan can improve carbohydrate and lipid metabolism, as well as control insulin resistance without causing the side effects that are associated with full PPARγ activators.
Migration of CD4-positive lymphocytes into the vessel wall represents an important step in early atherogenesis. Telmisartan is an angiotensin type 1 receptor (AT1R) blocker with peroxisome proliferator-activated receptor (PPAR)-gamma-activating properties. The present study examined the effect of telmisartan on CD4-positive cell migration and the role of PPARgamma in this context. CD4-positive lymphocytes express both the AT1R and PPARgamma. Stimulation of CD4-positive lymphocytes with stromal cell-derived factor (SDF)-1 leads to a 4.1+/-3.1-fold increase in cell migration. Pretreatment of cells with telmisartan reduces this effect in a concentration-dependent manner to a maximal 1.6+/-0.7-fold induction at 10 mumol/L of telmisartan (P<0.01 compared with SDF-1-treated cells; n=22). Three different PPARgamma activators, rosiglitazone, pioglitazone, and GW1929, had similar effects, whereas eprosartan, a non-PPARgamma-activating AT1R blocker, did not affect chemokine-induced lymphocyte migration. Telmisartan's effect on CD4-positive lymphocyte migration was mediated through an early inhibition of chemokine-induced phosphatidylinositol 3-kinase activity. Downstream, telmisartan inhibited F-actin formation, as well as intercellular adhesion molecule-3 translocation. Transfection of CD4-positive lymphocytes with PPARgamma small interfering RNA abolished telmisartan's effect on migration, whereas blockade of the AT1R had no such effect. Telmisartan inhibits chemokine-induced CD4-positive cell migration independent of the AT1R via PPARgamma. These data provide a novel mechanism to explain how telmisartan modulates lymphocyte activation by its PPARgamma-activating properties.
PMID:18158351 Walcher D et al; Hypertension 51 (2): 259-66 (2008)
Angiotensin II is formed from angiotensin I in a reaction catalyzed by angiotensin-converting enzyme (ACE, kininase II). Angiotensin II is the principal pressor agent of the renin-angiotensin system, with effects that include vasoconstriction, stimulation of synthesis and release of aldosterone, cardiac stimulation, and renal reabsorption of sodium. Telmisartan blocks the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT1 receptor in many tissues, such as vascular smooth muscle and the adrenal gland. Its action is therefore independent of the pathways for angiotensin II synthesis. There is also an AT2 receptor found in many tissues, but AT2 is not known to be associated with cardiovascular homeostasis. Telmisartan has much greater affinity (>3,000 fold) for the AT1 receptor than for the AT2 receptor. Blockade of the renin-angiotensin system with ACE inhibitors, which inhibit the biosynthesis of angiotensin II from angiotensin I, is widely used in the treatment of hypertension. ACE inhibitors also inhibit the degradation of bradykinin, a reaction also catalyzed by ACE. Because telmisartan does not inhibit ACE (kininase II), it does not affect the response to bradykinin. Whether this difference has clinical relevance is not yet known. Telmisartan does not bind to or block other hormone receptors or ion channels known to be important in cardiovascular regulation. Blockade of the angiotensin II receptor inhibits the negative regulatory feedback of angiotensin II on renin secretion, but the resulting increased plasma renin activity and angiotensin II circulating levels do not overcome the effect of telmisartan on blood pressure.
NIH; DailyMed. Current Medication Information for MIicardis (Telmisartan) Tablet (Revised: October 2012). Available from, as of October 9, 2014: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=cfb9309f-e0df-4a55-9542-0e869fce05fb
... The receptor-independent protective role of an angiotensin II receptor 1 blocker (ARB) /was investigated/ using primary-cultured mesangial cells from angiotensin II receptor 1 knockout or wild-type mice and a highly lipophilic ARB, telmisartan. Intracellular reactive oxygen species were estimated using a fluorogenic probe, CM-H2DCFDA. Non-angiotensin II-induced reactive oxygen species production was generated by exposing cells to hydrogen peroxide alone or after treatment with telmisartan. Flow cytometry analysis showed that angiotensin II induced an increase in oxidant production in a dose-dependent manner in wild-type cells, but not in knockout cells. In contrast, hydrogen peroxide induced oxidative stress in both wild-type and knockout cells. Interestingly, telmisartan attenuated the oxidative stress induced by hydrogen peroxide in both cells, suggesting that it acted via a receptor-independent antioxidant effect. Intracellular concentrations of telmisartan were confirmed by high-performance liquid chromatography analysis. Expression of plasminogen activator inhibitor 1, which is stimulated by oxidative stress, was also attenuated by telmisartan in a receptor-independent as well as receptor-dependent manner. Telmisartan did not change expression levels of antioxidative enzymes such as catalase or glutathione peroxidase. Furthermore, the amelioration of oxidative stress by telmisartan did not involve the peroxisome proliferator-activated receptor-gamma pathway. Telmisartan inhibits intracellular oxidative stress, at least in part, in a receptor-independent manner, possibly owing to its lipophilic and antioxidant structure.
PMID:17620961 Shao J et al; J Hypertens 25 (8): 1643-9 (2007)
Telmisartan, an angiotensin II type 1 receptor (AT1R) antagonist, was found to have a unique property: it is a partial agonist of peroxisome proliferator-activated receptor gamma (PPARgamma). ... /This study/ examined whether telmisartan affects AT1R expression in vascular smooth muscle cells ... derived from the thoracic aorta of Wistar-Kyoto rat. ... Telmisartan decreased the expression of AT1R at the mRNA and protein levels in a dose- and time-dependent manner. Decreased AT1R promoter activity with unchanged mRNA stability suggested that telmisartan suppressed AT1R gene expression at the transcriptional level. However, the expression of AT1R was not suppressed by other AT1R antagonists such as candesartan or olmesartan. Since the suppression of AT1R expression was prevented by pretreatment with GW9662, a PPARgamma antagonist, PPARgamma should have participated in the process. The deletion and mutation analysis of the AT1R gene promoter indicated that a GC box located in the proximal promoter region is responsible for the telmisartan-induced downregulation. Data provides a novel insight into an effect of telmisartan: telmisartan inhibits AT1R gene expression through PPARgamma activation. The dual inhibition of angiotensin II function by telmisartan - AT1R blockade and downregulation - would contribute to more complete inhibition of the renin-angiotensin system.
PMID:16938288 Imayama I et al; Cardiovasc Res 72 (1): 184-90 (2006)








