



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
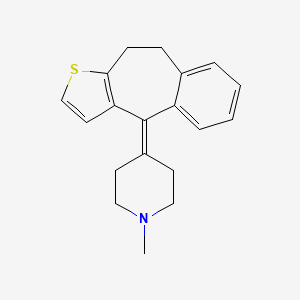

1. Bc 105
2. Bc-105
3. Bc105
4. Pizotyline
5. Polomigran
6. Sandomigran
1. 15574-96-6
2. Pizotyline
3. Sandomigran
4. Litec
5. Sandomygran
6. Bc-105
7. Pizotyline [usan]
8. Pizotifenum
9. Pizotifene
10. Pizotifeno
11. Bc 105
12. Pizotifan
13. 4-(9,10-dihydro-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ylidene)-1-methylpiperidine
14. Pizotifen [inn]
15. 4-(9,10-dihydro-4h-benzo[4,5]cyclohepta[1,2-b]thien-4-ylidene)-1-methylpiperidine
16. Piperidine, 4-(9,10-dihydro-4h-benzo(4,5)cyclohepta(1,2-b)thien-4-ylidene)-1-methyl-
17. Chebi:50212
18. 0by8440v3n
19. Pizotifen (inn)
20. Sanomigran
21. Pizotyline (usan)
22. 4-(9,10-dihydro-4h-benzo(4,5)cyclohepta(1,2-b)thien-4-ylidene)-1-methylpiperidine
23. Piperidine, 4-(9,10-dihydro-4h-benzo[4,5]cyclohepta[1,2-b]thien-4-ylidene)-1-methyl-
24. Ncgc00163169-01
25. Sandomigran (tn)
26. Dsstox_cid_3490
27. Dsstox_rid_77051
28. Dsstox_gsid_23490
29. Pizotifene [inn-french]
30. Pizotifenum [inn-latin]
31. Pizotifeno [inn-spanish]
32. 1-methyl-4-{6-thiatricyclo[8.4.0.0^{3,7}]tetradeca-1(10),3(7),4,11,13-pentaen-2-ylidene}piperidine
33. Cas-15574-96-6
34. Einecs 239-632-9
35. Brn 0753534
36. Pizotylline
37. Sandomigram
38. Sanmigran
39. Unii-0by8440v3n
40. 4-[9,10-dihydro-4h-benzo[4,5]cyclohepta[1,2-b]thien-4-ylidene]-1-methylpiperidine
41. Mfcd00864192
42. Pizotyline [mi]
43. Biomol-nt_000102
44. Gtpl93
45. Pizotifen [mart.]
46. Pizotifen [who-dd]
47. Oprea1_684518
48. Schembl44122
49. Mls006011927
50. Pizotyline Maleate (salt/mix)
51. Bpbio1_001391
52. Chembl294951
53. Zinc1968
54. Dtxsid6023490
55. Pizotifen, >=98% (hplc)
56. Bdbm82088
57. Bcp14794
58. Hy-b0115
59. Tox21_112024
60. Bbl009911
61. Nsc_27400
62. Pdsp1_001602
63. Pdsp2_001586
64. S5770
65. Stk801341
66. Akos005612964
67. Tox21_112024_1
68. Cs-1870
69. Db06153
70. Sb43212
71. Ncgc00163169-02
72. Ncgc00163169-03
73. Ncgc00163169-05
74. 1-methyl-4-(6-thiatricyclo[8.4.0.03,7]tetradeca-1(14),3(7),4,10,12-pentaen-2-ylidene)piperidine
75. Ac-11996
76. As-13328
77. Bp164276
78. Smr004703517
79. Sbi-0206788.p001
80. Cas_15574-96-6
81. Db-043288
82. Ab00514749
83. Ft-0637249
84. Ft-0655575
85. P2344
86. D05523
87. T71852
88. Ab00514749_11
89. Ab00514749_12
90. 574p966
91. L000722
92. Q413784
93. W-108016
94. Brd-k75958195-001-01-7
95. Brd-k75958195-037-03-7
96. Brd-k75958195-037-04-5
97. 4-(9,10-dihydro-1-thiabenzo[f]azulen-4-ylidene)-1-methylpiperidine
98. 4-oxo-9,10-dihydro-4h-benzo(4,5) Cyclohepto(1,2,b)thiophene
99. 4-oxo-9,10-dihydro-4h-benzo(4,5) Cyclohepto(1,2,b) Thiophene
100. 4-(4,5-dihydrobenzo[1,2]cyclohepta[2,4-b]thiophen-10-ylidene)-1-methylpiperidine
101. 1-methyl-4-(6-thiatricyclo[8.4.0.0^{3,7]tetradeca-1(14),3(7),4,10,12-pentaen-2-ylidene)piperidine
102. 1-methyl-4-{6-thiatricyclo[8.4.0.0?,?]tetradeca-1(14),3(7),4,10,12-pentaen-2-ylidene}piperidine
103. 1-methyl-4-{6-thiatricyclo[8.4.0.0^{3,7}]tetradeca-1(14),3(7),4,10,12-pentaen-2-ylidene}piperidine
104. 4-(9,10-dihydro-4h-benzo[4,5]cyclohepta[1,2-b]thiophen-4-ylidene)-1-methylpiperidine (c4h6o5)
| Molecular Weight | 295.4 g/mol |
|---|---|
| Molecular Formula | C19H21NS |
| XLogP3 | 3.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 295.13947085 g/mol |
| Monoisotopic Mass | 295.13947085 g/mol |
| Topological Polar Surface Area | 31.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 406 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the prophylactic management of migraines.
Various studies have shown pizotifen to be effective in the prophylaxis of migraines in reducing the frequency and severity of vascular headaches. Evidence from studies _in vivo_ and _in vitro_ demonstrate antagonistic actions towards serotonin and histamine. Pizotifen blocks the postsynaptic 5-HT2 receptors, as supported by antagonism of several direct agonists of 5-HT receptors. It is an antagonist at histamine H1 receptors, and is weakly anticholinergic. It also binds to 1- and 2-adrenergic receptors, and dopamine receptors. Pizotifen elicits a minimal effect as an epinephrine or bradykinin antagonist. Pizotifen exhibits weak sedative properties in mouse and monkey studies, as indicated by inhibition of locomotion and potentiation of barbiturates, without changes in cardiac or respiratory rates. In dogs, intravenous administration of pizotifen cause rapid hypotension but was reversed to normal within 30 minutes. Pizotifen was shown to inhibit serotonin uptake in the isolated perfused cat spleen and, _in vivo_, inhibits serotonin-induced contractions in rat uterus and cat nictiating membrane. In contrast, pizotifen demonstrated a venoconstrictor activity _in vivo_ when orally or intravenously administered to saphenous veins in conscious dogs. Pizotifen has the potential to stimulate the appetite and may cause weight gain upon treatment. In a double-blind clinical study of patients with mild to moderate depression, treatment of pizotifen led to clinical improvement of the depressive symptoms. However, deterioration of the schizophrenic emotional symptoms was also observed in patients with depression and chronic schizophrenia. This indicates that pizotifen may potentially improve the symptoms of patients with depressions in conjunction with migraines. Neuroprotective effect of pizotifen was investigated _in vitro_ in a mouse cell model of Huntington's disease (HD). According to a chemical screen of a mouse HdhQ111/Q111 striatal cell model of HD, treatment of pizotifen was associated with increased ATP levels and decreased activation of caspase-3, leading to enhanced cell viability. Transient activation of ERK signalling pathway lasting for less than 3 hours was also observed. In the R6/2 transgenic mouse model of HD, rotarod performance of the mouse treated with pizotifen was seen, accompanied by an increase in DARPP-32 protein expression and restoration of striatal area. However these effects being reflected _in vivo_ are not established.
Serotonin Antagonists
Drugs that bind to but do not activate serotonin receptors, thereby blocking the actions of serotonin or SEROTONIN RECEPTOR AGONISTS. (See all compounds classified as Serotonin Antagonists.)
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Analgesics, Non-Narcotic
A subclass of analgesic agents that typically do not bind to OPIOID RECEPTORS and are not addictive. Many non-narcotic analgesics are offered as NONPRESCRIPTION DRUGS. (See all compounds classified as Analgesics, Non-Narcotic.)
N - Nervous system
N02 - Analgesics
N02C - Antimigraine preparations
N02CX - Other antimigraine preparations
N02CX01 - Pizotifen
Absorption
The absorption half-life of pizotifen following oral administration is 0.5 to 0.8 hours in an adult male with nearly complete absorption rate of 80%. Maximum blood levels are reached 5 hours post-administration and the absolute bioavailability is 78%.
Route of Elimination
About one third of the total orally administered dose is excreted into the feces. Less than 1% of the total dose is excreted in the urine as the unchanged parent drug, and up to 55% of the dose is excreted as its metabolites.
Volume of Distribution
The volume of distribution in an adult male is 833L for pizotifen and 70L for the N-glucuronide conjugate.
Pizotifen is extensively metabolized in the liver, where it primarily undergoes N-glucuronidation to form the main metabolite, N-glucuronide conjugate. N-glucuronide conjugate accounts for at least 50% of the plasma and 60-70% of the urinary-excreted radioactivity.
The elimination half-life for pizotifen and N-glucuronide conjugate is about 23 hours.
While the mechanism of action is not fully understood, it is proposed that pizotifen works by inhibiting the peripheral actions of serotonin and histamine in increasing the membrane permeability of cranial vessels and transudation of plasmakinin, while altering pain thresholds in migraines. By blocking 5-HT receptors, pizotifen attenuates the signalling of serotonin in causing cranial vasoconstriction, as well as serotonin-enhanced platelet function and aggregation. There is evidence that it also inhibits the peripheral actions of bradykinin. Pizotifen may inhibit serotonin reuptake by blood platelets, which affects the tonicity and decreases passive distension of extracranial arteries. The effects of pizotifen leading to appetite stimulation may be due to the drug acting at the metabolic level rather than a direct stimulation of the appetite centre.


