



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0

1. Mebaral
2. Methylphenobarbital
3. Methylphenobarbitone
4. N-methylphenobarbital
5. Prominal
1. Methylphenobarbital
2. Mephobarbitone
3. Methylphenobarbitone
4. Metylfenemal
5. Enphenemal
6. Mebaral
7. Prominal
8. N-methylphenobarbital
9. Isonal
10. 115-38-8
11. Menta-bal
12. Enfenemal
13. Mephytal
14. Morbusan
15. Phemetone
16. Phemiton
17. Phemitone
18. Metyna
19. Methyl-calminal
20. 1-methylphenobarbital
21. N-methylphenolbarbitol
22. Methylphenolbarbital
23. Methylphenobarbitonum
24. Methylphenylbarbituric Acid
25. Isonal (roussel)
26. Methylphenobarbitalum
27. Metilfenobarbital
28. Metilfenobarbitale [dcit]
29. 5-ethyl-1-methyl-5-phenylbarbituric Acid
30. N-ethylmethylphenylbarbituric Acid
31. N-methylethylphenylbarbituric Acid
32. Methyl Phenobarbitone
33. Metilfenobarbital [inn-spanish]
34. Methylphenobarbitalum [inn-latin]
35. 1-methyl-5-ethyl-5-phenylbarbituric Acid
36. 5-ethyl-1-methyl-5-phenyl-1,3-diazinane-2,4,6-trione
37. N-methyl-5-phenyl-5-ethylbarbital
38. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5-ethyl-1-methyl-5-phenyl-
39. 5-ethyl-n-methyl-5-phenylbarbituric Acid
40. 1-methyl-5-phenyl-5-ethylbarbituric Acid
41. 5-phenyl-5-ethyl-3-methylbarbituric Acid
42. Mephobarbital Civ
43. 5-ethyl-5-phenyl-n-methyl-bartituric Acid
44. 5-ethyl-1-methyl-5-phenyl-2,4,6(1h,3h,5h)-pyrimidinetrione
45. Barbituric Acid, 5-ethyl-1-methyl-5-phenyl-
46. 5-ethyl-1-methyl-5-phenylpyrimidine-2,4,6(1h,3h,5h)-trione
47. Enphenemalum
48. Methylphenobarbital (inn)
49. Methylphenobarbital [inn]
50. 5nc67nu76b
51. Chebi:6758
52. Prominaletten
53. Prominalum
54. Ncgc00159357-02
55. Meberal
56. Mephobarbital [jan]
57. Metilfenobarbitale
58. 5-ethyl-1-methyl-5-phenyl-pyrimidine-2,4,6-trione
59. Mebaral (tn)
60. Hsdb 3579
61. Einecs 204-085-7
62. Mephobarbital (jan/usp)
63. Mephobarbital [usp:jan]
64. Brn 0256144
65. Unii-5nc67nu76b
66. Dea No. 2250
67. N-methyl-5-phenyl-5-ethylbarbituric Acid
68. Dsstox_cid_3258
69. Phenobarbital, Mono-methyl
70. Mephobarbital [mi]
71. (.+/-.)-mephobarbital
72. Cambridge Id 5240602
73. Dsstox_rid_76944
74. Mephobarbital [hsdb]
75. Dsstox_gsid_23258
76. Oprea1_133475
77. Schembl35624
78. Mephobarbital [vandf]
79. 5-24-09-00294 (beilstein Handbook Reference)
80. Chembl45029
81. Dtxsid4023258
82. Alarqzqtbtvljv-uhfffaoysa-
83. Mephobarbital Civ [usp-rs]
84. Methylphenobarbital [mart.]
85. Methylphenobarbital [who-dd]
86. Tox21_111600
87. Mephobarbital [usp Monograph]
88. Stk732046
89. Akos002254670
90. Db00849
91. Ncgc00159357-03
92. Cas-115-38-8
93. Methylphenobarbital [ep Monograph]
94. Methylphenobarbital 0.1 Mg/ml In Methanol
95. Methylphenobarbital 1.0 Mg/ml In Methanol
96. C07829
97. D00700
98. 5-ethyl-1-methyl-5-phenyl-barbituric Acid
99. A803425
100. Q411697
101. 1-methyl-5-ethyl-5-phenyl-pyrimidine-2,4,6-trione
102. Hexahydropyrimidine-2,4,6-trione,1-methyl-5-ethyl-5-phenyl-
103. Mephobarbital, United States Pharmacopeia (usp) Reference Standard
104. Methylphenobarbital, European Pharmacopoeia (ep) Reference Standard
105. Methylphenobarbital, 1.0 Mg/ml In Methanol, Certified Reference Material
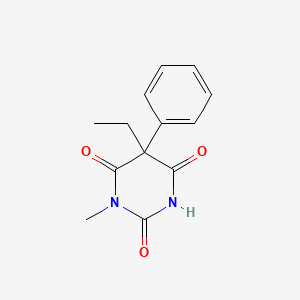
| Molecular Weight | 246.26 g/mol |
|---|---|
| Molecular Formula | C13H14N2O3 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 246.10044231 g/mol |
| Monoisotopic Mass | 246.10044231 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 388 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Anticonvulsants; GABA Modulators; Sedatives, Barbiturate
National Library of Medicine's Medical Subject Headings online file (MeSH, 2009)
Mephobarbital is indicated for use as a sedative for the relief of anxiety, tension, and apprehension, and as an anticonvulsant for the treatment of grand mal and petit mal epilepsy.
Novak, K.M. (ed.). Drug Facts and Comparisons2008 Edition. Wolters Kluwer Health. St. Louis, Missouri 2008., p. 1444
/Certain barbiturates, including mephobarbital/ ... have also been used for routine sedation to relieve anxiety, tension, and apprehension; however, barbiturates generally have been replaced by benzodiazepines for daytime sedation. /Included in US product label/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 501
/EXPERIMENTAL THERAPY/ In order to find a drug for the prevention of metabolic hyperbilirubinemia of the newborn, which has less sedative effects than phenobarbital (PB) the effect of methylphenobarbital (MPB) on the plasma bilirubin concentration of newborns was studied in a double blind trial. MPB (3 x 10 mg/kg on the first day) reduced the plasma bilirubin level in mature newborns on day four by 33% in comparison to those on placebo. The results justify further investigations in premature babies, who frequently suffer from disturbances which may facilitate the development of bilirubin encephalopathy.
PMID:3241289 Grauel L et al; J Perinat Med 16 (5-6): 431-5 (1988).
Mephobarbital shares the toxic potentials of the barbiturate-derivative anticonvulsants, and the usual precautions of anticonvulsant administration should be observed.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2582
Clinicians should inform patients, their families, and caregivers about the potential for an increased risk of suicidal thinking and behavior (suicidality) associated with anticonvulsant therapy.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2582
Adverse effects of barbiturates may include drowsiness, lethargy, vertigo, headache, severe CNS depression, mental depression, and myalgic, neuralgic, or arthralgic pain. Residual sedation or "hangover" occurs frequently following hypnotic doses, and subtle distortion of mood, impaired judgment, and impaired motor skills may persist for many hours. Some patients, particularly those with severe pain, experience paradoxical excitement and/or euphoria, restlessness, or delirium, and, therefore, barbiturates should not be administered in the presence of uncontrolled pain. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2577
Geriatric patients may react to usual doses of barbiturates with excitement, confusion, or depression. Many patients experience increased dreaming, nightmares, or increased insomnia when hypnotic doses of barbiturates are discontinued. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2577
For more Drug Warnings (Complete) data for Methylphenobarbital (28 total), please visit the HSDB record page.
The toxic dose of barbiturates varies considerably but, in general, a severe reaction is likely to occur when the amount ingested is more than 10 times the usual oral hypnotic dose. Potentially lethal blood concentrations are those in excess of 80 ug/mL for phenobarbital, 50 ug/mL for amobarbital or butabarbital, and approximately 30 ug/mL for secobarbital or pentobarbital; however, some patients have survived much higher blood concentrations. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2578
For the relief of anxiety, tension, and apprehension, also used as an anticonvulsant for the treatment of epilepsy.
Methylphenobarbital, a barbiturate, is used in combination with acetaminophen or aspirin and caffeine for its sedative and relaxant effects in the treatment of tension headaches, migraines, and pain. Barbiturates act as nonselective depressants of the central nervous system (CNS), capable of producing all levels of CNS mood alteration from excitation to mild sedation, hypnosis, and deep coma. In sufficiently high therapeutic doses, barbiturates induce anesthesia.
GABA Modulators
Substances that do not act as agonists or antagonists but do affect the GAMMA-AMINOBUTYRIC ACID receptor-ionophore complex. GABA-A receptors (RECEPTORS, GABA-A) appear to have at least three allosteric sites at which modulators act: a site at which BENZODIAZEPINES act by increasing the opening frequency of GAMMA-AMINOBUTYRIC ACID-activated chloride channels; a site at which BARBITURATES act to prolong the duration of channel opening; and a site at which some steroids may act. GENERAL ANESTHETICS probably act at least partly by potentiating GABAergic responses, but they are not included here. (See all compounds classified as GABA Modulators.)
Hypnotics and Sedatives
Drugs used to induce drowsiness or sleep or to reduce psychological excitement or anxiety. (See all compounds classified as Hypnotics and Sedatives.)
Anticonvulsants
Drugs used to prevent SEIZURES or reduce their severity. (See all compounds classified as Anticonvulsants.)
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AA - Barbiturates and derivatives
N03AA01 - Methylphenobarbital
Absorption
Approximately 50% of an oral dose of mephobarbital is absorbed from the gastrointestinal tract.
Approximately 50% of an oral dose of mephobarbital is absorbed from the GI tract. Plasma concentrations required for therapeutic effects are unknown.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2582
Barbiturates are absorbed in varying degrees following oral, rectal, or im administration. The sodium salts are more rapidly absorbed by all routes of administration than are the acids. The rate of oral absorption is increased when the sodium salt is ingested as a dilute solution or taken on an empty stomach. Alcohol also enhances the rate of absorption, possibly by increasing blood flow through the gastric mucosa. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Following oral or rectal administration, the onset of action varies from ... 20-60 minutes for metharbital, mephobarbital, and phenobarbital. IM administration results in a slightly faster onset of action. Following iv administration of the sodium salts of amobarbital, pentobarbital, phenobarbital, or secobarbital, the onset of action ranges from almost immediately for methohexital, pentobarbital, and thiopental to 5 minutes for phenobarbital. Maximum effects of thiopental or pentobarbital are achieved within about 1 minute while as much as 30 minutes may be required with administration of phenobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
The duration of sedative effects of all the barbiturates is usually 3-6 hours following iv administration and 6-8 hours when the drugs are administered by other routes. There appears to be very little difference in duration of hypnotic action among barbiturates used orally as hypnotics. Therefore, most authorities now believe that barbiturates should be classified according to their intended pharmacologic action (ie, sedative-hypnotic barbiturates and anesthetic barbiturates [methohexital, thiamylal (no longer commercially available in the US), thiopental]), rather than as long-acting (mephobarbital, metharbital, and phenobarbital), intermediate-acting (amobarbital and butabarbital), short-acting (aprobarbital, pentobarbital, and secobarbital), and ultrashort-acting (methohexital, thiopental). /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Absorption, Distribution and Excretion (Complete) data for Methylphenobarbital (15 total), please visit the HSDB record page.
Hepatic, primarily by the hepatic microsomal enzyme system. About 75% of a single oral dose of mephobarbital is metabolized to phenobarbital in 24 hours.
The principal route of mephobarbital metabolism is N-demethylation by the liver to form phenobarbital. About 75% of a single oral dose of mephobarbital is converted to phenobarbital in 24 hours. Chronic administration of mephobarbital leads to accumulation of phenobarbital (not mephobarbital) in plasma. It has not been definitely determined whether mephobarbital contributes to the anticonvulsant effect or whether the metabolite, phenobarbital, is the only active agent during mephobarbital therapy. Phenobarbital may be excreted in the urine unchanged, as the p-hydroxyphenobarbital metabolite, or as glucuronide or sulfate conjugates. Alkalinization of the urine and/or increasing the urine flow substantially increases the rate of excretion of unchanged phenobarbital.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2582
Barbiturates are slowly metabolized, chiefly by hepatic microsomal enzymes. Phenobarbital and probably other barbiturates induce hepatic microsomal enzymes and thus may accelerate metabolism of other concomitantly administered drugs metabolized by these enzymes. There is no conclusive evidence that barbiturates accelerate their own metabolism. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
It has not been established whether mephobarbital, like phenobarbital, is a potent inducer of the enzymes involved in the metabolism of other drugs, but because the drug is chemically and pharmacologically similar to phenobarbital in addition to being metabolized to phenobarbital, this possibility is likely.
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2582
The stereoselectivity of the metabolism and pharmacokinetics of methylphenobarbital (MPB) was studied in six healthy adult male volunteers given single oral doses of the racemic drug. All of the volunteers were phenotypically extensive metabolizers of the drug. The R- and S-enantiomers of MPB were analyzed in plasma by an enantioselective HPLC method, and the enantiomers of the 4-hydroxy-MPB metabolite in urine by a similar procedure. The (R)-MPB was extensively hydroxylated, with an average of 49.56% of that enantiomer being recovered in urine as (R)-4-hydroxy-MPB. Only 7.16% of the (S)-MPB was converted to the corresponding hydroxy metabolite. The extensive hydroxylation of (R)-MPB resulted in rapid elimination of this enantiomer, with a terminal plasma half-life of 7.52 +/- 1.70 (SD) hr. The (S)-MPB, the only recognized metabolites of which were (S)-4-hydroxy-MPB and phenobarbital (PB), was eliminated very slowly [t1/2, 69.78 +/- 14.77 (SD) hr]. The oral clearance of (R)-MPB (0.470 +/- 0.184 (SD) liters/hr/kg) was much higher than that of (S)-MPB [0.017 +/- 0.001 (SD) liters/hr/kg]. The extreme differences in metabolic fate and pharmacokinetics of the enantiomers of MPB are interesting. Most of the circulating PB seemed to be derived from (S)-MPB. ...
PMID:2565213 Lim WH, Hooper WD; Drug Metab Dispos 17 (2): 212-7 (1989).
For more Metabolism/Metabolites (Complete) data for Methylphenobarbital (6 total), please visit the HSDB record page.
34 (range 11-67) hours
The plasma half-life of the barbiturates ranges from 1.5-22 hours for methohexital and thiopental to 2-6 days for phenobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
...The extensive hydroxylation of (R)-MPB resulted in rapid elimination of this enantiomer, with a terminal plasma half-life of 7.52 +/- 1.70 (SD) hr. The (S)-MPB, the only recognized metabolites of which were (S)-4-hydroxy-MPB and phenobarbital (PB), was eliminated very slowly [t1/2, 69.78 +/- 14.77 (SD) hr]. ...
PMID:2565213 Lim WH, Hooper WD; Drug Metab Dispos 17 (2): 212-7 (1989).
Methylphenobarbital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged.
The exact mechanism(s) by which barbiturates exert their effect on the CNS, has not been fully elucidated. However, it is believed that such effects are related, at least partially, to the drugs' ability to enhance the activity of gamma-aminobutyric acid (GABA), the principal inhibitory neurotransmitter in the CNS, by altering inhibitory synaptic transmissions that are mediated by Alpha-GABA receptors. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Although the drugs act throughout the CNS, a site of particular sensitivity is the polysynaptic midbrain reticular formation which is concerned with the arousal mechanism. Barbiturates induce an imbalance in central inhibitory and facilitatory mechanisms influencing the cerebral cortex and the reticular formation. The significance of the effect of barbiturates on neurotransmitters is unclear. It appears that the drugs decrease the excitability of both presynaptic and postsynaptic membranes. It has not been determined which of the various actions of barbiturates at cellular and synaptic levels are responsible for their sedative and hypnotic effects. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Relatively low doses of the barbiturates depress the sensory cortex, decrease motor activity, and produce sedation and drowsiness. In some patients, however, drowsiness may be preceded by a period of transient elation, confusion, euphoria, or excitement, especially after subhypnotic doses of aprobarbital, pentobarbital, or secobarbital. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
Larger doses distort judgment, cloud perception, suppress motor activity, and produce drowsiness and sleep. Still larger doses induce anesthesia. Barbiturate-induced sleep differs from physiologic sleep. Barbiturates reduce the rapid eye movement (REM) or dreaming stage of sleep. Stages III and IV sleep are also decreased. Although tolerance develops to the REM-suppressant effects during chronic administration, REM rebound occurs when the drugs are withdrawn, and the patient may experience markedly increased dreaming, nightmares, and/or insomnia. /Barbiturates General Statement/
American Society of Health System Pharmacists; AHFS Drug Information 2009. Bethesda, MD. (2009), p. 2579
For more Mechanism of Action (Complete) data for Methylphenobarbital (12 total), please visit the HSDB record page.


