



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
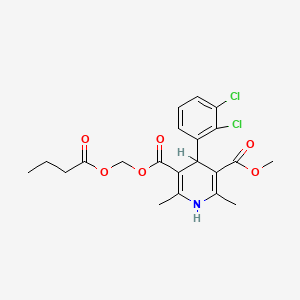

1. Butyroxymethyl Methyl 4-(2',3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
2. Cleviprex
1. 167221-71-8
2. Clevidipine Butyrate
3. Cleviprex
4. Clevelox
5. Clevidepine
6. Clevidepine Butyrate
7. 166432-28-6
8. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, 3-methyl 5-[(1-oxobutoxy)methyl] Ester
9. Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dime Thyl-3,5-pyridinedicarboxylate
10. 19o2gp3b7q
11. 3-((butyryloxy)methyl) 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
12. 5-o-(butanoyloxymethyl) 3-o-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
13. Methyl (1-oxobutoxy)methyl 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylate
14. Rac-clevidipine
15. H324/38
16. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, Methyl (1-oxobutoxy)methyl Ester
17. Methyl 5-{[(butanoyloxy)methoxy]carbonyl}-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-carboxylate
18. H-324/38
19. (butanoyloxy)methyl Methyl (4rs)-4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
20. Sr-01000945183
21. Clevidipine [usan:inn]
22. Unii-19o2gp3b7q
23. Cleviprex (tn)
24. 3,5-pyridinedicarboxylic Acid, 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-, 3-methyl 5-((1-oxobutoxy)methyl) Ester
25. 3-[(butyryloxy)methyl] 5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
26. Clevidipine [inn]
27. H 324/38
28. Cleviprex (clevidipine)
29. Clevidipine [mi]
30. Clevidipine (usan/inn)
31. Clevidipine [usan]
32. Clevidepine [vandf]
33. Clevidipine [vandf]
34. Clevidipine [mart.]
35. Dsstox_cid_31450
36. Dsstox_rid_97336
37. Dsstox_gsid_57661
38. Clevidipine [who-dd]
39. Schembl115522
40. Gtpl7468
41. Clevidipine Butyrate;clevidipine
42. Chembl1237132
43. Dtxsid6057661
44. Clevidipine [orange Book]
45. Chebi:135738
46. Hms3604h21
47. Hms3651g10
48. Hms3884l03
49. Amy22141
50. Bcp22676
51. Bcp22687
52. Ex-a2898
53. Clevidepine Butyrate [vandf]
54. Clevidipine Butyrate [vandf]
55. Tox21_113923
56. Bdbm50088387
57. Mfcd00940070
58. S2080
59. Akos015896325
60. Ccg-269294
61. Cs-1427
62. Db04920
63. Ncgc00262928-01
64. 4-(2,3-dichlorophenyl)-1,4-dihydro-2,6-dimethyl-3,5-pyridinedicarboxylic Acid 3-methyl 5-[(1-oxobutoxy)methyl] Ester
65. Ac-24370
66. As-19935
67. Butyroxymethyl Methyl 4-(2',3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
68. Clevidipine Butyrate [orange Book]
69. Hy-17436
70. Db-064636
71. C3503
72. Cas-167221-71-8
73. Ft-0659562
74. Ft-0688416
75. Sw220087-1
76. D08892
77. Ab01566888_01
78. 221c718
79. A810832
80. J-520103
81. Q5132338
82. Sr-01000945183-1
83. Sr-01000945183-2
84. Z1691545244
85. 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic Aicd 3-[(butyryloxy)methyl] 5-methyl Ester
86. Butyroxymethyl Methyl 4-(2',3'-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylat
87. O3-(butanoyloxymethyl) O5-methyl 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
| Molecular Weight | 456.3 g/mol |
|---|---|
| Molecular Formula | C21H23Cl2NO6 |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Exact Mass | 455.0902428 g/mol |
| Monoisotopic Mass | 455.0902428 g/mol |
| Topological Polar Surface Area | 90.9 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 748 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Cleviprex |
| PubMed Health | Clevidipine (Injection) |
| Drug Classes | Antihypertensive |
| Drug Label | Cleviprex is a sterile, milky-white emulsion containing 0.5 mg/mL of clevidipine suitable for intravenous administration. Clevidipine is a dihydropyridine calcium channel blocker. Chemically, the active substance, clevidipine, is butyroxymethyl methy... |
| Active Ingredient | Clevidipine |
| Dosage Form | Emulsion |
| Route | Intravenous |
| Strength | 125mg/250ml (0.5mg/ml); 25mg/50ml (0.5mg/ml); 50mg/100ml (0.5mg/ml) |
| Market Status | Prescription |
| Company | Medicines |
| 2 of 2 | |
|---|---|
| Drug Name | Cleviprex |
| PubMed Health | Clevidipine (Injection) |
| Drug Classes | Antihypertensive |
| Drug Label | Cleviprex is a sterile, milky-white emulsion containing 0.5 mg/mL of clevidipine suitable for intravenous administration. Clevidipine is a dihydropyridine calcium channel blocker. Chemically, the active substance, clevidipine, is butyroxymethyl methy... |
| Active Ingredient | Clevidipine |
| Dosage Form | Emulsion |
| Route | Intravenous |
| Strength | 125mg/250ml (0.5mg/ml); 25mg/50ml (0.5mg/ml); 50mg/100ml (0.5mg/ml) |
| Market Status | Prescription |
| Company | Medicines |
For the reduction of blood pressure when when oral antihypertensive therapy is not feasible or not desirable.
FDA Label
Treatment of hypertensive disease
Clevidipine belongs to a well-known class of drugs called dihydropyridine calcium channel antagonists. Clevidpine is the first third generation intravenous dihydropyridine calcium channel blocker. In vitro studies demonstrated that clevidipine acts by selectively relaxing the smooth muscle cells that line small arteries, resulting in arterial dilation, widening of the artery opening, and without reducing central venous pressure or reducing cardiac output.
Calcium Channel Blockers
A class of drugs that act by selective inhibition of calcium influx through cellular membranes. (See all compounds classified as Calcium Channel Blockers.)
C08CA16
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
C - Cardiovascular system
C08 - Calcium channel blockers
C08C - Selective calcium channel blockers with mainly vascular effects
C08CA - Dihydropyridine derivatives
C08CA16 - Clevidipine
Route of Elimination
urine 63-74%, feces 7-22%
Clevidipine is rapidly hydrolyzed to inactive metabolites by esterases in arterial blood.
1 minute
Possibly by deforming the channel, inhibiting ion-control gating mechanisms, and/or interfering with the release of calcium from the sarcoplasmic reticulum, clevidipine inhibits the influx of extracellular calcium across both the myocardial and vascular smooth muscle cell membranes. The resultant inhibition of the contractile processes of the myocardial smooth muscle cells leads to dilation of the coronary and systemic arteries and improved oxygen delivery to the myocardial tissue.




