
Synopsis
Synopsis
0
USDMF
0
CEP/COS
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Canada
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
0
News #PharmaBuzz
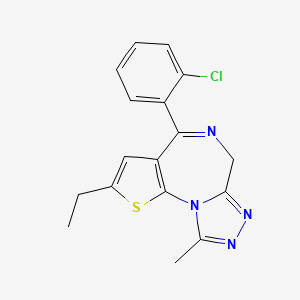

1. Etizolam, 14c-labeled
2. Y 7131
3. Y-7131
1. 40054-69-1
2. Depas
3. 4-(2-chlorophenyl)-2-ethyl-9-methyl-6h-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine
4. Sedekopan
5. Y-7131
6. A76xi0hl37
7. 4-(o-chlorophenyl)-2-ethyl-9-methyl-6h-thieno(3,2-f)-s-triazolo(4,3-a)(1,4)diazepine
8. Ahr3219;y7131
9. Ncgc00182031-01
10. Etizolamum
11. Etizolam [inn:jan]
12. Etizolamum [inn-latin]
13. Ahr 3219
14. 7-(2-chlorophenyl)-4-ethyl-13-methyl-3-thia-1,8,11,12-tetraazatricyclo[8.3.0.0^{2,6}]trideca-2(6),4,7,10,12-pentaene
15. Brn 0572740
16. Unii-a76xi0hl37
17. Sedekopan (tn)
18. Etizolam [inn]
19. Etizolam [jan]
20. Etizolam [mi]
21. Etizolam (jp17/inn)
22. Dsstox_cid_3030
23. Etizolam [mart.]
24. Etizolam [who-dd]
25. 6-(o-chlorophenyl)-8-ethyl-1-methyl-4h-s-triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine
26. 6-(o-chlorphenyl)-8-aethyl-1-methyl-4h-s-triazolo(3,4-c)thieno(2,3-e)(1,4)diazepin [german]
27. 6h-thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine, 4-(2-chlorophenyl)-2-ethyl-9-methyl-
28. 8-ethyl-6-(o-chlorophenyl)-1-methyl-4h-s-triazolo(3,4c)thieno(2,3e)-1,4-diazepine
29. Dsstox_rid_76838
30. Dsstox_gsid_23030
31. Schembl42920
32. Zinc1402
33. Chembl1289779
34. Dtxsid0023030
35. Chebi:31583
36. Ahr3219
37. Etizolam 0.1 Mg/ml In Methanol
38. Etizolam 1.0 Mg/ml In Methanol
39. 4c66
40. Hms3652n06
41. Bcp22893
42. Hy-b0677
43. Tox21_112931
44. S4276
45. Akos022185397
46. Db09166
47. Ds-3112
48. 4h-s-triazolo(3,4-c)thieno(2,3-e)(1,4)-diazepine, 6-(o-chlorophenyl)-8-ethyl-1-methyl-
49. 6-(o-chlorphenyl)-8-aethyl-1-methyl-4h-s-triazolo(3,4-c)thieno(2,3-e)(1,4)diazepin
50. Ncgc00182031-05
51. Cas-40054-69-1
52. Sw219903-1
53. A14257
54. D01514
55. Q409966
56. Sr-01000883960
57. Sr-01000883960-1
58. Etizolam Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
59. 4-(2-chlorophenyl)-2-ethyl-9-methyl-6h-thieno[3,2-f] [1,2,4]triazolo[4,3-a] [1,4]diazepine
60. 4-(2-chlorophenyl)-2-ethyl-9-methyl-6h-thieno[3,2-f][1,2,4]-triazolo[4,3-a][1,4]diazepine
61. 7-(2-chlorophenyl)-4-ethyl-13-methyl-3-thia-1,8,11,12-tetraazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaene
62. 7-(2-chlorophenyl)-4-ethyl-13-methyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaene
63. H4c
| Molecular Weight | 342.8 g/mol |
|---|---|
| Molecular Formula | C17H15ClN4S |
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 2 |
| Exact Mass | 342.0705954 g/mol |
| Monoisotopic Mass | 342.0705954 g/mol |
| Topological Polar Surface Area | 71.3 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 474 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for the treatment of generalized anxiety disorder with depression, panic disorder and insomnia.
Etizolam is a CNS depressant with anxiolytic, anticonvulsant, sedative-hypnotic and muscle relaxant effects. It acts on the benzodiazepine site of the GABA-A receptor as an agonist to increase inhibitory GABAergic transmission throughout the central nervous system. Studies indicate that etizolam mediates its pharmacological actions with 6 to 10 times more potency than that of diazepam. Clinical human studies performed in Italy showed clinical effectiveness of etizolam in relieving symptoms in patients with generalized anxiety disorders with depressive symptoms. Etizolam also mediates imipramine-like neuropharmacological and behavioral effects, as well as minor effects on cognitive functioning. It is shown to substitute the actions of a short-acting barbiturate, pentobarbitol, in a drug discrimination study. Etizolam is an antagonist at platelet-activating-factor (PAF) receptor and attenuates the recurrence of chronic subdural hematoma after neurosurgery in clinical studies. It is shown to inhibit PAF-induced bronchoconstriction and hypotension.
Tranquilizing Agents
A traditional grouping of drugs said to have a soothing or calming effect on mood, thought, or behavior. Included here are the ANTI-ANXIETY AGENTS (minor tranquilizers), ANTIMANIC AGENTS, and the ANTIPSYCHOTIC AGENTS (major tranquilizers). These drugs act by different mechanisms and are used for different therapeutic purposes. (See all compounds classified as Tranquilizing Agents.)
N - Nervous system
N05 - Psycholeptics
N05B - Anxiolytics
N05BA - Benzodiazepine derivatives
N05BA19 - Etizolam
Absorption
Etizolam is well absorbed from the intestines with a biological bioavailability of 93% following oral administration. After a single oral dosing of 0.5mg etizolam, it takes approximately 0.9 hours to reach the peak plasma concentration of 8.3 ng/mL.
Route of Elimination
In a rat study, the amounts of etizolam excreted was 30% in urine was 70% in feces, while the values in a mouse study were 40% in urine and 60% in feces.
Volume of Distribution
Apparent distribution volume was 0.9 0.2 L/kg following a single oral doing of 0.5mg etizolam.
Biotransformation of etizolam is extensive and involves hydroxylation and conjugation. The main metabolite formed via 1'-hydroxylation is -hydroxyetizolam which retains pharmacological activity comparable to that of the parent drug, indicating that the action of metabolites may contribute to the clinical effects of etizolam. CYP3A4 is predicted to be the main CYP enzyme responsible for mediating etizolam metabolism. CYP2C18 and CYP2C19 are also involved in the metabolic pathways.
Etizolam has known human metabolites that include 7-(2-chlorophenyl)-4-ethyl-13-methyl-3-thia-1,8,11,12-tetrazatricyclo[8.3.0.02,6]trideca-2(6),4,7,10,12-pentaen-9-ol and alpha-Hydroxyetizolam.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The average elimination half life of etizolam following a single oral dose of 0.5mg is 3.4 hours but may be increased up to 17 hours depending on the rate of metabolism. The main metabolite -hydroxyetizolam displays a longer elimination half life of 8.2 hours.
Etizolam is selectively a full agonist at GABA-A receptors to increase GABAergic transmission and enhance GABA-induced Cl- currents. It is reported to bind to the benzodiazepine binding site which is located across the interface between the alpha and gamma subunits. Benzodiazapines are reported to only bind to receptors that contain gamma 2 and alpha 1/2/3/5 subunits. Alpha-1-containing receptors mediate the sedative effects of etizolam whereas alpha-2 and alpha-3 subunit-containing receptors mediate the anxiolytic effect. Etizolam shows high potency and affinity towards GABA-A receptor with alpha 1 beta 2 gamma 2S subunit combination. By binding to the regulatory site of the receptor, etizolam potentiates GABA transmission by facilitating the opening of GABA-induced chloride channels. Etizolam is a specific antagonist at PAFR. It inhibits PAF-induced platelet aggregation by inhibiting PAF binding to the receptors located on the surface of platelets with an IC50 of 22nM.




Registration Number : 217MF11220
Registrant's Address : 121-15 Torino, Koka-cho, Koka City, Shiga Prefecture
Initial Date of Registration : 2005-12-12
Latest Date of Registration :




Registration Number : 222MF10191
Registrant's Address : 1978-96 Ogushi, Ube City, Yamaguchi Prefecture
Initial Date of Registration : 2010-07-07
Latest Date of Registration :


Registration Number : 225MF10155
Registrant's Address : “CENTAUR HOUSE”, NEAR GRAND HYATT, SHANTI NAGAR, VAKOLA, SANTACRUZ (EAST), MUMBAI-400 055, INDIA
Initial Date of Registration : 2013-08-12
Latest Date of Registration :

Date of Issue : 2025-08-19
Valid Till : 2028-06-25
Written Confirmation Number : WC-0107
Address of the Firm :

Registrant Name : Chong Kun Dang Co., Ltd.
Registration Date : 2021-07-11
Registration Number : 20210711-209-J-1055
Manufacturer Name : Centaur Pharmaceuticals Private Limited
Manufacturer Address : Plot No. 75, 76, 76/1 & 74 Chikhloli MIDC Ambernath (West), Thane - 421 501, Maharashtra State, India



Registration Number : 218MF11013
Registrant's Address : Via Curiel 34, 20067 Paulo, Milano, ITALY
Initial Date of Registration : 2006-12-18
Latest Date of Registration :


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
(2-amino-5-ethylthiophen-3-yl)(2-chlorophenyl) met...
CAS Number : 50508-60-6
End Use API : Etizolam
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

2-(Aminoacetylamino)-3-(o-chlorobenzoyl)-5-ethylth...
CAS Number : 50509-09-6
End Use API : Etizolam
About The Company : Beijing Cooperate Pharmaceutical Co.,Ltd is a high-tech company specializing in pharmaceutical and advanced material, involving API,pharmaceutical intermediates...

CAS Number : 43183-98-8
End Use API : Etizolam
About The Company : KARPSCHEM.SOLUTIONS is an emerging, research-based global pharmaceutical company with a diverse combination of skills, resources, and capabilities that provide ...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Global Sales Information

Market Place

ANALYTICAL

ABOUT THIS PAGE
40
PharmaCompass offers a list of Etizolam API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Etizolam manufacturer or Etizolam supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Etizolam manufacturer or Etizolam supplier.
PharmaCompass also assists you with knowing the Etizolam API Price utilized in the formulation of products. Etizolam API Price is not always fixed or binding as the Etizolam Price is obtained through a variety of data sources. The Etizolam Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A DSSTox_CID_3030 manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of DSSTox_CID_3030, including repackagers and relabelers. The FDA regulates DSSTox_CID_3030 manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. DSSTox_CID_3030 API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of DSSTox_CID_3030 manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A DSSTox_CID_3030 supplier is an individual or a company that provides DSSTox_CID_3030 active pharmaceutical ingredient (API) or DSSTox_CID_3030 finished formulations upon request. The DSSTox_CID_3030 suppliers may include DSSTox_CID_3030 API manufacturers, exporters, distributors and traders.
click here to find a list of DSSTox_CID_3030 suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The DSSTox_CID_3030 Drug Master File in Japan (DSSTox_CID_3030 JDMF) empowers DSSTox_CID_3030 API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the DSSTox_CID_3030 JDMF during the approval evaluation for pharmaceutical products. At the time of DSSTox_CID_3030 JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of DSSTox_CID_3030 suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a DSSTox_CID_3030 Drug Master File in Korea (DSSTox_CID_3030 KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of DSSTox_CID_3030. The MFDS reviews the DSSTox_CID_3030 KDMF as part of the drug registration process and uses the information provided in the DSSTox_CID_3030 KDMF to evaluate the safety and efficacy of the drug.
After submitting a DSSTox_CID_3030 KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their DSSTox_CID_3030 API can apply through the Korea Drug Master File (KDMF).
click here to find a list of DSSTox_CID_3030 suppliers with KDMF on PharmaCompass.
A DSSTox_CID_3030 written confirmation (DSSTox_CID_3030 WC) is an official document issued by a regulatory agency to a DSSTox_CID_3030 manufacturer, verifying that the manufacturing facility of a DSSTox_CID_3030 active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting DSSTox_CID_3030 APIs or DSSTox_CID_3030 finished pharmaceutical products to another nation, regulatory agencies frequently require a DSSTox_CID_3030 WC (written confirmation) as part of the regulatory process.
click here to find a list of DSSTox_CID_3030 suppliers with Written Confirmation (WC) on PharmaCompass.
DSSTox_CID_3030 Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of DSSTox_CID_3030 GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right DSSTox_CID_3030 GMP manufacturer or DSSTox_CID_3030 GMP API supplier for your needs.
A DSSTox_CID_3030 CoA (Certificate of Analysis) is a formal document that attests to DSSTox_CID_3030's compliance with DSSTox_CID_3030 specifications and serves as a tool for batch-level quality control.
DSSTox_CID_3030 CoA mostly includes findings from lab analyses of a specific batch. For each DSSTox_CID_3030 CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
DSSTox_CID_3030 may be tested according to a variety of international standards, such as European Pharmacopoeia (DSSTox_CID_3030 EP), DSSTox_CID_3030 JP (Japanese Pharmacopeia) and the US Pharmacopoeia (DSSTox_CID_3030 USP).

