



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
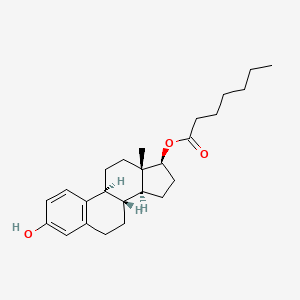

1. 4956-37-0
2. Oestradiol 17-heptanoate
3. Estradiol Enantate
4. Estradiol 17-heptanoate
5. Estradiol Enanthate [usan]
6. [(8r,9s,13s,14s,17s)-3-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl] Heptanoate
7. Sq 16,150
8. Pap315wzia
9. Sq-16150
10. So-16150
11. Sq-16,150
12. Estradiol Enanthate (usan)
13. Unii-pap315wzia
14. Ncgc00188442-01
15. Estradiol Heptanoate
16. Einecs 225-599-8
17. B-estradiol 17-enanthate
18. Dsstox_cid_3001
19. Dsstox_rid_76826
20. Beta-estradiol 17-enanthate
21. Dsstox_gsid_23001
22. Beta-estradiol 17-heptanoate
23. Mls006010232
24. Schembl547096
25. 17beta-estradiol 17-enanthate
26. Chembl1697792
27. Dtxsid3023001
28. Estra-1,3,5(10)-triene-3,17beta-diol 17-heptanoate
29. Chebi:135604
30. Estradiol Enantate [mart.]
31. Amy22313
32. Bcp28275
33. Estradiol Enantate [who-dd]
34. Hy-b1830
35. Zinc4215835
36. Tox21_113032
37. Estra-1,3,5(10)-triene-3,17-diol (17.beta.)-, 17-heptanoate
38. Mfcd00056542
39. Akos015918000
40. Estradiol 17-heptanoate [mi]
41. Ds-2760
42. Smr004701310
43. Cas-4956-37-0
44. So 16,150
45. Cs-0013900
46. E1151
47. D04064
48. 956e370
49. A827747
50. Q5401763
51. Estra-1,3,5(10)-triene-3,17b-diol 17-heptanoate
52. N'-(imino(5-nitrofuran-2-yl)methyl)propionohydrazide
53. (17?)-3-hydroxyestra-1,3,5(10)-trien-17-yl Heptanoate
54. (17beta)-3-hydroxyestra-1(10),2,4-trien-17-yl Heptanoate
55. Estra-1,3,5(10)-triene-3,17-diol (17beta)-17-heptanoate
56. Estra-1,3,5(10)-triene-3,17-diol (17beta)-, 17-heptanoate
57. (1s,10r,11s,14s,15s)-5-hydroxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-yl Heptanoate
| Molecular Weight | 384.6 g/mol |
|---|---|
| Molecular Formula | C25H36O3 |
| XLogP3 | 7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 7 |
| Exact Mass | 384.26644501 g/mol |
| Monoisotopic Mass | 384.26644501 g/mol |
| Topological Polar Surface Area | 46.5 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 546 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Estrogens
Compounds that interact with ESTROGEN RECEPTORS in target tissues to bring about the effects similar to those of ESTRADIOL. Estrogens stimulate the female reproductive organs, and the development of secondary female SEX CHARACTERISTICS. Estrogenic chemicals include natural, synthetic, steroidal, or non-steroidal compounds. (See all compounds classified as Estrogens.)


