



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
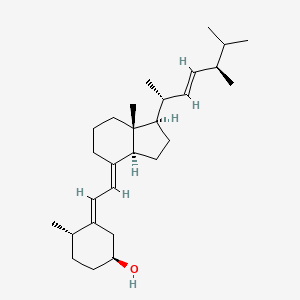

1. At 10
2. At-10
3. At10
4. Calcamine
5. Dihydrotachysterin
6. Tachystin
1. Dichystrolum
2. Dihidrotaquisterol
3. 67-96-9
4. Anti-tetany Substance 10
5. Dihydrotachysterolum
6. Calcamine
7. At 10
8. Dihydrotachysterol2
9. Antitanil
10. Dihydral
11. Hytakerol
12. Tachyrol
13. Tachystin
14. Dihydrotachysterol 2
15. Dygratyl
16. Parterol
17. Chebi:4591
18. Dht2
19. R5lm3h112r
20. Dihydro Tachysterol
21. Tachysterol, Dihydro-
22. Tachysterol2, Dihydro-
23. Dichysterol
24. (3s,5e,7e,10s,22e)-9,10-secoergosta-5,7,22-trien-3-ol
25. (5e,7e,22e)-(3s,10s)-9,10-seco-5,7,22-ergostatrien-3-ol
26. Diidrotachisterolo
27. A.t. 10
28. Dihydrotachysterol2 / (5e)-(10s)-10,19-dihydrovitamin D2 / (5e)-(10s)-10,19-dihydroergocalciferol
29. 9,10-secoergosta-5,7,22-trien-3beta-ol
30. Dht(sub 2)
31. Dihydrotachysterol(sub 2)
32. Diidrotachisterolo [dcit]
33. Unii-r5lm3h112r
34. Dihidrotaquisterol [inn-spanish]
35. Dihydrotachysterolum [inn-latin]
36. Hytakerol (tn)
37. Ncgc00166147-02
38. Einecs 200-672-7
39. Dihydrotachysterol [usp:inn:ban:jan]
40. 9,10-secoergosta-5,7,22-trien-3.beta.-ol
41. 9,10-secoergosta-5,7,22-trien-3-ol, (3beta,5e,7e,10alpha,22e)-
42. Dsstox_cid_2938
43. Dsstox_rid_76796
44. Dsstox_gsid_22938
45. Schembl41340
46. Tachysterol Dihydro Derivative
47. Dihydrotachysterol [mi]
48. Dihydrotachysterol [inn]
49. Dihydrotachysterol [jan]
50. 9,10-secoergosta-5,7,22-trien-3-ol, (3.beta.,5e,7e,10.alpha.,22e)-
51. Chembl2356023
52. Dtxsid5022938
53. Dihydrotachysterol [hsdb]
54. Dihydrotachysterol [mart.]
55. Dihydrotachysterol (jan/usp/inn)
56. Dihydrotachysterol [usp-rs]
57. Dihydrotachysterol [who-dd]
58. Cyclohexanol, 4-methyl-3-((2e)-2-((1r,3as,7ar)-octahydro-7a-methyl-1-((1r,2e,4r)-1,4,5-trimethyl-2-hexen-1-yl)-4h-inden-4-ylidene)ethylidene)-, (1s,3e,4s)-
59. Hy-a0245
60. Zinc4212953
61. Tox21_112341
62. Lmst03010056
63. Db01070
64. Cas-67-96-9
65. Dihydrotachysterol [ep Impurity]
66. Dihydrotachysterol [ep Monograph]
67. Dihydrotachysterol [usp Impurity]
68. Tachysterol Dihydro Derivative [mi]
69. Cs-0017590
70. D00299
71. Q155685
| Molecular Weight | 398.7 g/mol |
|---|---|
| Molecular Formula | C28H46O |
| XLogP3 | 7.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 1 |
| Rotatable Bond Count | 5 |
| Exact Mass | 398.354866087 g/mol |
| Monoisotopic Mass | 398.354866087 g/mol |
| Topological Polar Surface Area | 20.2 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 639 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 7 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Bone Density Conservation Agents; Vitamins
National Library of Medicine, SIS; ChemIDplus Record for Dihydrotachysterol (67-96-9). Available from, as of March 15, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
MEDICATION (VET): Calcium regulator used in veterinary medicine to treat hypercalcemia.
Milne, G.W.A. Veterinary Drugs: Synonyms and Properties. Ashgate Publishing Limited, Aldershot, Hampshire, England 2002., p. 109
Dihydrotachysterol is indicated ... for treatment of chronic and latent forms of post operative tetany and idiopathic tetany. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2966
Therapeutic doses of specific vitamin D analogs are used in the treatment of chronic hypocalcemia, hypophosphatemia, rickets, and osteodystrophy associated with various medical conditions including chronic renal failure, familial hypophosphatemia, and hypoparathyroidism (postsurgical or idiopathic, or pseudohypoparathyroidism). Some analogs have been found to reduce elevated parathyroid hormone concentrations in patients with renal osteodystrophy associated with hyperparathyroidism. Theoretically, any of the vitamin D analogs may be used for the above conditions, However, because of their pharmacologic properties, some may be more useful in certain situations than others. Alfacalcidol, calcitriol, and dihydrotachysterol are usually preferred in patients with renal failure since these patients have impaired ability to synthesize calcitriol from cholecalciferol and ergocalciferol; therefore, the response is more predictable. In addition, their shorter half-lives may make toxicity easier to manage (hypercalcemia reverses more quickly). Ergocalciferol may not be the preferred agent in the treatment of familial hypophosphatemia or hypoparathyroidism because the large doses needed are associated with a risk of overdose and hypercalcemia; dihydrotachysterol and calcitriol may be preferred. /Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2966
/Dihydrotachysterol/...should not be used in presence of renal insufficiency or hyperphosphatemia. Extreme care must be used to prevent overdosage.
Osol, A. (ed.). Remington's Pharmaceutical Sciences. 16th ed. Easton, Pennsylvania: Mack Publishing Co., 1980., p. 919
Doses of vitamin D analogs that do not exceed the physiologic requirement are usually nontoxic. However, some infants and patients with sarcoidosis or hypoparathyroidism may have increased sensitivity to vitamin D analogs. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Acute or chronic administration of excessive doses of vitamin D analogs or enhanced responsiveness to physiologic amounts of ergocalciferol or cholecalciferol may lead to hypervitaminosis D manifested by hypercalcemia. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
Decreased renal function without hypercalcemia has also been reported in patients with hypoparathyroidism after long-term vitamin D analog therapy. Before therapy with vitamin D analogs is initiated, serum phosphate concentrations must be controlled. To avoid ectopic calcification, the serum calcium (in mg/dL) times phosphorus (in mg/dL) should not be allowed to exceed 70. Because administration of vitamin D analogs may increase phosphate absorption, patients with renal failure may require adjustment in the dosage of aluminum-containing antacids used to decrease phosphate absorption. /Vitamin D analogs/
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 3541
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
Used for the prevention and treatment of rickets or osteomalacia, and to manage hypocalcemia associated with hypoparathyroidism or pseudohypoparathyroidism. Also used for the treatment of vitamin D dependent rickets, rickets or osteomalacia secondary to long-term high dose anticonvulsant therapy, early renal osteodystrophy, osteoporosis (in conjunction with calcium), and hypophosphatemia associated with Fanconi syndrome (with treatment of acidosis).
Dihydrotachysterol is hydroxylated in the liver to 25-hydroxydihydrotachysterol, which is the major circulating active form of the drug. It does not undergo further hydroxylation by the kidney and therefore is the analogue of 1, 25-dihydroxyvitamin D. Dihydrotachysterol is effective in the elevation of serum calcium by stimulating intestinal calcium absorption and mobilizing bone calcium in the absence of parathyroid hormone and of functioning renal tissue. Dihydrotachysterol also increases renal phosphate excretion.
Vitamins
Organic substances that are required in small amounts for maintenance and growth, but which cannot be manufactured by the human body. (See all compounds classified as Vitamins.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
A - Alimentary tract and metabolism
A11 - Vitamins
A11C - Vitamin a and d, incl. combinations of the two
A11CC - Vitamin d and analogues
A11CC02 - Dihydrotachysterol
Onset of action: Hypercalcemic: Several hours (maximal after 1 to 2 weeks).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
...Major path of elimination of dihydrotachysterol & its metabolites is probably secretion into bile & excretion in feces ... dihydrotachysterol /is also/ excreted in breast milk ...
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1976
Duration of action: following oral administration: Up to 9 weeks.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 2967
The primary route of excretion of vitamin D is the bile; only a small percentage of an administered dose is found in urine. /Vitamin D/
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1730
For more Absorption, Distribution and Excretion (Complete) data for DIHYDROTACHYSTEROL (8 total), please visit the HSDB record page.
DHT3 /vitamin D3/ undergoes 25-hydroxylation to yield 25-hydroxydihydrotachysterol3 (25-OHDHT3), which appears to be active form of DHT (dihydrotachysterol) in both intestine & bone.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 1582
High specific radioactivity (14)C- and (3)H-labeled dihydrotachysterol were prepared and their metabolites studied in rachitic chicks and rats. 20% dihydrotachysterol was excreted in the bile in the first 24 hours, about 50% as a carboxylic acid derivative. No hydroxylation at C-1 was observed, although polar metabolites were detected in all tissues. Larger proportions of the parent steroid and its 25-OH derivative were detected in tissues compared with cholecalciferol but no single metabolite was detected at the intracellular site of action of cholecalciferol.
Lawson DE M, Bell PA; Biochem J 142(1) 37 (1974)
Absorbed vitamin D circulates in blood in association with vitamin D-binding protein, which is a specific alpha-globulin. Vitamin D disappears from plasma with /a half-life/ of 19 to 25 hr, but it is stored in body for prolonged periods (6 months or longer in rat) apparently in fat deposits throughout body. /Vitamin D/
Gilman, A. G., L. S. Goodman, and A. Gilman. (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 6th ed. New York: Macmillan Publishing Co., Inc. 1980., p. 1543
Once hydroxylated to 25-hydroxydihydrotachysterol, the modified drug binds to the vitamin D receptor. The bound form of the vitamin D receptor serves as a transcriptional regulator of bone matrix proteins, inducing the expression of osteocalcin and suppressing synthesis of type I collagen. Vitamin D (when bound to the vitamin D receptor)stimulates the expression of a number of proteins involved in transporting calcium from the lumen of the intestine, across the epithelial cells and into blood. This stimulates intestinal calcium absorption and increases renal phosphate excretion. These are functions that are normally carried out by the parathyroid hormone.
Elevates serum calcium concentration by increasing intestinal absorption of calcium & possibly by enhancing urinary excretion of inorganic phosphate... /drug-induced/ phosphaturia may be ... due to increased serum calcium level and its effect on phosphate clearance by kidneys.
American Society of Hospital Pharmacists. Data supplied on contract from American Hospital Formulary Service and other current ASHP sources., p. 1976


