



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
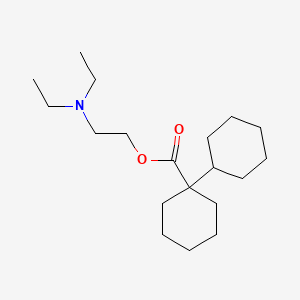

1. Bentyl
2. Bentylol
3. Di Cyclonex
4. Di Spaz
5. Di-cyclonex
6. Di-spaz
7. Dibent
8. Diclomin
9. Dicyclomine Hydrochloride
10. Dicycloverin
11. Hydrochloride, Dicyclomine
12. Lomine
13. Merbentyl
14. Or Tyl
15. Or-tyl
16. Spascol
1. Dicycloverine
2. Dicycloverin
3. 77-19-0
4. Bentyl
5. Diocyl
6. Di-syntramine
7. Bentylol
8. Dicicloverina
9. Wyovin
10. Dicycloverinum
11. Dicymine
12. Procyclomin
13. Bentomine
14. Mamiesan
15. Sawamin
16. Atumin
17. Dyspas
18. 2-(diethylamino)ethyl 1-cyclohexylcyclohexane-1-carboxylate
19. Oxityl-p
20. Kolantyl
21. Diocyl Hydrochloride
22. Wyovin Hydrochloride
23. Dicycloverin Hydrochloride
24. Dicycloverine (inn)
25. [1,1'-bicyclohexyl]-1-carboxylic Acid, 2-(diethylamino)ethyl Ester
26. (bicyclohexyl)-1-carboxylic Acid, 2-(diethylamino)ethyl Ester
27. (1,1'-bicyclohexyl)-1-carboxylic Acid, 2-(diethylamino)ethyl Ester
28. 4kv4x8if6v
29. Chembl1123
30. 2-(diethylamino)ethyl 1-cyclohexylcyclohexanecarboxylate
31. 2-(diethylamino)ethyl 1,1'-bi(cyclohexyl)-1-carboxylate
32. Chebi:4514
33. Bicyclohexyl-1-carboxylic Acid 2-diethylamino-ethyl Ester
34. Ncgc00015368-06
35. Diethylaminocarbethoxybicyclohexyl Hydrochloride
36. Dicycloverinum [inn-latin]
37. Dicicloverina [inn-spanish]
38. Dsstox_cid_2926
39. Dicycloverine [inn]
40. Dsstox_rid_76790
41. Dsstox_gsid_22926
42. Bentyl Hydrochloride
43. Bentylol; Dicyclomine; Dicycloverin; Dicycloverine
44. Bentylol Hydrochloride
45. Dicycloverine [inn:ban]
46. Cas-77-19-0
47. Dicymine (tn)
48. Dicyclomine [inn]
49. Hsdb 3058
50. Einecs 201-009-4
51. Unii-4kv4x8if6v
52. Bicyclohexyl-1-carbonsaeure-2'diethylaminoethylester
53. Byclomine
54. [bicyclohexyl]-1-carboxylic Acid, 2-(diethylamino)ethyl Ester
55. Bis(cyclohexyl)carboxylic Acid Diethylaminoethyl Ester Hydrochloride
56. 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate
57. Spectrum_000934
58. Dicyclomine [mi]
59. Prestwick0_000048
60. Prestwick1_000048
61. Prestwick2_000048
62. Prestwick3_000048
63. Spectrum2_000590
64. Spectrum3_000388
65. Spectrum4_000509
66. Spectrum5_000873
67. Lopac-d-7909
68. Dicyclomine [hsdb]
69. Dicyclomine [vandf]
70. Dicycloverine (dicyclomine)
71. Schembl3317
72. Lopac0_000432
73. Bspbio_000175
74. Bspbio_002175
75. Gtpl355
76. Kbiogr_001057
77. Kbioss_001414
78. Divk1c_000162
79. Spbio_000440
80. Spbio_002096
81. Dicycloverine [who-dd]
82. Bpbio1_000193
83. Dtxsid1022926
84. Curutkgfnzgfse-uhfffaoysa-
85. Hy-b1339a
86. Kbio1_000162
87. Kbio2_001414
88. Kbio2_003982
89. Kbio2_006550
90. Kbio3_001395
91. Ninds_000162
92. Hms3604h12
93. Zinc1530613
94. Tox21_113571
95. 2-diethylaminoethyl 1-cyclohexylcyclohexane-1-carboxylate Hydrochloride
96. Bdbm50010101
97. Nsc404381
98. Stl356799
99. [bicyclohexyl]-1-carboxylic Acid, 2-(diethylamino)ethyl Ester Hydrochloride
100. Akos022107181
101. Tox21_113571_1
102. Ccg-204524
103. Db00804
104. Sdccgsbi-0050417.p005
105. Cas-67-92-5
106. Idi1_000162
107. Ncgc00015368-01
108. Ncgc00015368-02
109. Ncgc00015368-03
110. Ncgc00015368-04
111. Ncgc00015368-05
112. Ncgc00015368-07
113. Ncgc00015368-08
114. Ncgc00015368-11
115. Ncgc00015368-17
116. Ncgc00016300-01
117. Ncgc00024386-03
118. Ncgc00024386-04
119. Sbi-0050417.p004
120. Ab00053456
121. Cs-0013567
122. Wln: L6tj A- Al6tj Avo2n2&2 &gh
123. (bicyclohexyl)-1-carboxylic Acid, Hydrochloride
124. C06951
125. D07820
126. [1, 2-(diethylamino)ethyl Ester, Hydrochloride
127. Ab00053456_14
128. Ab00053456_15
129. L000680
130. Q2662662
131. 2-diethylaminoethyl 1-cyclohexylcyclohexanecarboxylate
132. Brd-k68507560-003-05-5
133. Brd-k68507560-003-15-4
134. 2-(diethylamino)ethyl (bicyclohexyl)-1-carboxylate
135. .beta.-diethylaminoethyl-1-cyclohexylhexahydrobenzoate Hydrochloride
136. .beta.-diethylaminoethyl 1-cyclohexylcyclohexanecarboxylate Hydrochloride
137. 104959-55-9
| Molecular Weight | 309.5 g/mol |
|---|---|
| Molecular Formula | C19H35NO2 |
| XLogP3 | 5.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 8 |
| Exact Mass | 309.266779359 g/mol |
| Monoisotopic Mass | 309.266779359 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 326 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Bentyl |
| PubMed Health | Dicyclomine |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosageforms:Dicyclomine Hydrochloride Capsules, USP for oral use contain 10 mg dicyclomine hydrochloride, USP. Dicyclomine hydroch... |
| Active Ingredient | Dicyclomine hydrochloride |
| Dosage Form | Injectable; Tablet; Syrup; Capsule |
| Route | Injection; Oral |
| Strength | 10mg/5ml; 10mg; 10mg/ml; 20mg |
| Market Status | Prescription |
| Company | Aptalis Pharma Us; Forest Labs |
| 2 of 2 | |
|---|---|
| Drug Name | Bentyl |
| PubMed Health | Dicyclomine |
| Drug Classes | Gastrointestinal Agent |
| Drug Label | Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following dosageforms:Dicyclomine Hydrochloride Capsules, USP for oral use contain 10 mg dicyclomine hydrochloride, USP. Dicyclomine hydroch... |
| Active Ingredient | Dicyclomine hydrochloride |
| Dosage Form | Injectable; Tablet; Syrup; Capsule |
| Route | Injection; Oral |
| Strength | 10mg/5ml; 10mg; 10mg/ml; 20mg |
| Market Status | Prescription |
| Company | Aptalis Pharma Us; Forest Labs |
Muscarinic Antagonists; Parasympatholytics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
...OFTEN CLASSIFIED WITH ANTIMUSCARINIC AGENTS AS "ANTISPASMODICS," DO NOT PROPERLY BELONG TO GROUP OF ANTIMUSCARINIC AGENTS. ...DICYCLOMINE HYDROCHLORIDE, VSP-DECR SPASM OF GI TRACT, BILIARY TRACT, URETER, & UTERUS WITHOUT PRODUCING CHARACTERISTIC ATROPINIC EFFECTS ON SALIVARY, SWEAT, OR GI GLANDS, EYE, CV SYSTEM... /HCL/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 527
IT DECR MOTILITY BUT DOES NOT SUPPRESS GASTRIC SECRETION. IT IS USED IN TREATMENT OF IRRITABLE COLON, SPASTIC CONSTIPATION, MUCOUS COLITIS, SPASTIC COLITIS, PYLOROSPASM, & BILIARY DYSKINESIA. IN TREATMENT OF PEPTIC ULCER IT IS USED TO DELAY GASTRIC EMPTYING. /HYDROCHLORIDE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 844
ANTICHOLINERGIC /HYDROCHLORIDE/
The Merck Index. 9th ed. Rahway, New Jersey: Merck & Co., Inc., 1976., p. 409
DICYCLOMINE SHOULD BE USED CAUTIOUSLY IN PT WITH PROSTATIC HYPERTROPHY, BLADDER NECK OBSTRUCTION, PYLORIC OBSTRUCTION, & CARDIOSPASM. EVEN THOUGH IT DOES NOT APPEAR TO RAISE INTRAOCULAR PRESSURE IN NARROW-ANGLE GLAUCOMA, IT IS ADVISABLE TO MONITOR PRESSURE OF SUCH PT.
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 844
CLINICAL USE OF.../BENTYL/ HAS BEEN DISAPPOINTING.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 527
A 3-YR-OLD MALE INGESTED APPROX 100 TABLETS OF BENDECTIN & DEVELOPED TONIC-CLONIC SEIZURES FOLLOWED BY CARDIAC ARREST. ANALYSIS YIELDED HIGH LEVELS OF DOXYLAMINE, DICYCLOMINE & PYRIDOXINE. DOXYLAMINE APPEARS TO BE TOXIC CONSTITUENT.
BAYLEY M ET AL; FATAL OVERDOSE FROM BENDECTIN; CLIN PEDIATR (PHILA) 14: 507 (1975)
Dicyclomine is indicated for the treatment of functional bowel disorder and irritable bowel syndrome.
FDA Label
Dicyclomine is an anticholinergic drug used to relax the smooth muscles of the intestines. It's duration of action is not especially long as it is usually taken 4 times daily with individual doses of 20-40mg orally or 10-20mg by intramuscular injection. Dicyclomine should not be administered intravenously.
Parasympatholytics
Agents that inhibit the actions of the parasympathetic nervous system. The major group of drugs used therapeutically for this purpose is the MUSCARINIC ANTAGONISTS. (See all compounds classified as Parasympatholytics.)
Muscarinic Antagonists
Drugs that bind to but do not activate MUSCARINIC RECEPTORS, thereby blocking the actions of endogenous ACETYLCHOLINE or exogenous agonists. Muscarinic antagonists have widespread effects including actions on the iris and ciliary muscle of the eye, the heart and blood vessels, secretions of the respiratory tract, GI system, and salivary glands, GI motility, urinary bladder tone, and the central nervous system. (See all compounds classified as Muscarinic Antagonists.)
A - Alimentary tract and metabolism
A03 - Drugs for functional gastrointestinal disorders
A03A - Drugs for functional gastrointestinal disorders
A03AA - Synthetic anticholinergics, esters with tertiary amino group
A03AA07 - Dicycloverine
Absorption
The bioavailability of dicyclomine has not been determined, though it is likely well absorbed as the primary route of elimination is in the urine. Dicyclomine has a Tmax of 1-1.5h.
Route of Elimination
Dicyclomine is 79.5% eliminated in the urine and 8.4% in the feces.
Volume of Distribution
The volume of distribution for a 20mg oral dose is 3.65L/kg.
Clearance
Data regarding the clearance of dicyclomine is not readily available.
The metabolism of dicyclomine has not been well researched.
The mean plasma elimination half life is approximately 1.8 hours.
Dicyclomine achieves its action partially through direct antimuscarinic activity of the M1, M3, and M2 receptors; and partially through antagonism of bradykinin and histamine. Dicyclomine non-competitively inhibits the action of bradykinin and histamine, resulting in direct action on the smooth muscle, and decreased strength of contractions seen in spasms of the ileum.
...MAJOR ACTION APPEARS TO BE NONSPECIFIC DIRECT RELAXANT ACTION ON SMOOTH MUSLCE RATHER THAN COMPETITIVE ANTAGONISM OF ACH.
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 527






