



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
0
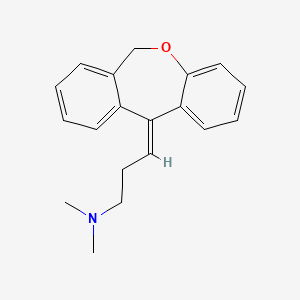

1. Apo Doxepin
2. Apo-doxepin
3. Apodoxepin
4. Aponal
5. Deptran
6. Desidox
7. Doneurin
8. Doxepia
9. Doxepin
10. Doxepin Beta
11. Doxepin Hydrochloride
12. Doxepin Hydrochloride, Cis-trans Isomer Mixture (approximately 1:5)
13. Doxepin Rph
14. Doxepin-rph
15. Espadox
16. Hydrochloride, Doxepin
17. Mareen
18. Novo Doxepin
19. Novo-doxepin
20. Prudoxin
21. Quitaxon
22. Sinquan
23. Xepin
24. Zonalon
1. Doxepin
2. (e)-doxepin
3. Trans-doxepin
4. 1668-19-5
5. Doxepin, (e)-
6. Doxepinum [inn-latin]
7. Doxepina [inn-spanish]
8. Sinequan (tn)
9. 3607-34-9
10. 851nlb57hq
11. Doxepin [usan]
12. Doxepina
13. Doxepinum
14. 11-(3-dimethylaminopropylidene)-6,11-dihydrodibenz(b,e)oxipin
15. Deptran
16. 1-propanamine, 3-dibenz(b,e)oxepin-11(6h)-ylidene-n,n-dimethyl-
17. Mf 10
18. (3e)-3-(dibenzo[b,e]oxepin-11(6h)-ylidene)-n,n-dimethylpropan-1-amine
19. Doxepin (inn)
20. [11c]doxepin
21. 3-(dibenzo[b,e]oxepin-11(6h)-ylidene)-n,n-dimethylpropan-1-amine
22. [11c]-doxepin
23. Ccris 9176
24. (3e)-3-(6h-benzo[c][1]benzoxepin-11-ylidene)-n,n-dimethylpropan-1-amine
25. Hsdb 3069
26. Doxepin [inn:ban]
27. Ncgc00015344-03
28. Unii-5asj6huz7d
29. Cas-1229-29-4
30. Unii-851nlb57hq
31. Methyllactate
32. 11-(3-(dimethylamino)propylidene)-6h-dibenz(b,e)oxepine
33. 11-(3-dimethylamino-propyliden)-6,11-dihydro-dibenz(b,e)oxipin
34. N,n-dimethyldibenz(b,e)oxepin-delta(11(6h),gamma)-propylamine
35. E-doxepin
36. Tocris-0508
37. P-3693a
38. Prestwick2_000263
39. Prestwick3_000263
40. Doxepin, E-isomer
41. Lopac-d-4526
42. 5asj6huz7d
43. Chembl860
44. Doxepin [usan:inn:ban]
45. Lopac0_000339
46. Bspbio_000106
47. Schembl116895
48. Bpbio1_000118
49. Gtpl1225
50. Gtpl3958
51. Zinc1331
52. (e)-3-(dibenzo[b,e]oxepin-11(6h)-ylidene)-n,n-dimethylpropan-1-amine
53. Hy-b0725a
54. Dtxsid90859605
55. (3e)-3-dibenzo(b,e)oxepin-11(6h)-ylidene-n,n-dimethylpropan-1-amine
56. Bdbm112780
57. Dibenz(b,e)oxepin-delta(sup 11(6h)),gamma-propylamine, N,n-dimethyl-
58. Ccg-204434
59. Sdccgsbi-0050327.p002
60. Ncgc00015344-01
61. Ncgc00015344-02
62. Ncgc00015344-04
63. Ncgc00015344-12
64. Ncgc00024623-01
65. Ncgc00162127-01
66. Us8629135, Sw-07
67. Cs-0013584
68. C06971
69. D07875
70. L000699
71. Brd-k36616567-003-01-5
72. Brd-k54462405-003-03-3
73. Brd-k54462405-003-16-5
74. Q27077103
75. 1-propanamine, 3-dibenz(b,e)oxepin-11(6h)-ylidene-n,n-dimethyl-, (3e)-
76. 5eh
| Molecular Weight | 279.4 g/mol |
|---|---|
| Molecular Formula | C19H21NO |
| XLogP3 | 4.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 279.162314293 g/mol |
| Monoisotopic Mass | 279.162314293 g/mol |
| Topological Polar Surface Area | 12.5 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 363 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Zonalon |
| PubMed Health | Doxepin |
| Drug Classes | Antianxiety, Antidepressant, Antiulcer, Dermatological Agent, Sleep Aid |
| Drug Label | Zonalon (doxepin hydrochloride) Cream, 5% is a topical cream. Each gram contains: 50 mg of doxepin hydrochloride (equivalent to 44.3 mg of doxepin).Doxepin hydrochloride is one of a class of agents known as dibenzoxepin tricyclic antidepressant com... |
| Active Ingredient | Doxepin hydrochloride |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Zonalon |
| PubMed Health | Doxepin |
| Drug Classes | Antianxiety, Antidepressant, Antiulcer, Dermatological Agent, Sleep Aid |
| Drug Label | Zonalon (doxepin hydrochloride) Cream, 5% is a topical cream. Each gram contains: 50 mg of doxepin hydrochloride (equivalent to 44.3 mg of doxepin).Doxepin hydrochloride is one of a class of agents known as dibenzoxepin tricyclic antidepressant com... |
| Active Ingredient | Doxepin hydrochloride |
| Dosage Form | Cream |
| Route | Topical |
| Strength | 5% |
| Market Status | Prescription |
| Company | Fougera Pharms |
Adrenergic Uptake Inhibitors; Anti-Anxiety Agents; Antidepressive Agents, Tricyclic; Antipruritics
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
A DIBENZOXEPIN DERIVATIVE THAT IS A PSYCHOTHERAPEUTIC AGENT WITH ANTIANXIETY & ANTIDEPRESSANT PROPERTIES. ...RECOMMENDED FOR MGMNT OF ANXIETY &/OR DEPRESSIVE STATES ASSOCIATED WITH PSYCHONEUROSIS, PSYCHOSIS, ALCOHOLISM, & ORG DISEASE. /HYDROCHLORIDE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1030
VET: FOR RELIEF OF PRURITIS IN DERMATOSES IN DOGS. MILD TRANQUILIZING EFFECT IS NOTED AFTER PROLONGED THERAPY OR ON DOSES ABOVE THOSE RECOMMENDED.
Rossoff, I.S. Handbook of Veterinary Drugs. New York: Springer Publishing Company, 1974., p. 189
Doxepin is indicated for the short-term (up to 8 days) topical treatment of moderate pruritus in adult patients with eczematous dermatitis, e.g., atopic dermatitis and lichen simplex chronicus. /Included in US product labeling; Doxepin, topical/
USP Convention. USPDI - Drug Information for the Health Care Professional. 17th ed. Volume I. Rockville, MD: Convention, Inc., 1997. (Plus Updates)., p. 1280
For more Therapeutic Uses (Complete) data for DOXEPIN (9 total), please visit the HSDB record page.
DOXEPIN HYDROCHLORIDE IS CONTRAINDICATED IN PT WITH GLAUCOMA OR TENDENCY TO URINARY RETENTION. ...SHOULD NOT BE ADMIN TO PT EITHER ON MAO INHIBITORS OR WHO HAVE BEEN...WITHIN THE PRIOR 2 WK. ...MAY...POTENTIATE DEPRESSANT EFFECT OF ALCOHOL. USE...IN PREGNANT PT OR...CHILDREN UNDER 12...NOT RECOMMENDED. /HYDROCHLORIDE/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 1030
ABOUT 2-3 WK MUST PASS BEFORE THERAPEUTIC EFFECTS...ARE EVIDENT. FOR THIS REASON, TRICYCLIC ANTIDEPRESSANTS SHOULD NEVER BE PRESCRIBED ON AN "AS-NEEDED" BASIS. ...SLOW ONSET OF EFFECTS MAY RELATE TO CHANGES IN METAB OF BIOGENIC AMINES THAT OCCUR... /IMIPRAMINE/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 175
...GENERAL USE FOR HYPNOSIS IS NOT RECOMMENDED. IN ADEQUATE DOSES THEY CAUSE HANGOVER & ARE NOT AS EFFECTIVE AS A CONVENTIONAL HYPNOTIC. /TRICYCLIC ANTIDEPRESSANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 175
OCCASIONAL PT WILL SHOW PHYSICAL DEPENDENCE ON TRICYCLIC ANTIDEPRESSANTS, WITH MALAISE, CHILLS, CORYZA, & MUSCLE ACHING FOLLOWING ABRUPT DISCONTINUATION OF HIGH DOSES... /IMIPRAMINE/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 441
For more Drug Warnings (Complete) data for DOXEPIN (25 total), please visit the HSDB record page.
4 OR 5. 4= VERY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) IS 50-500 MG/KG, BETWEEN 1 TEASPOON & 1 OUNCE FOR 70 KG PERSON (150 LB). 5= EXTREMELY TOXIC, PROBABLE ORAL LETHAL DOSE (HUMAN) IS 5-50 MG/KG, BETWEEN 7 DROPS & 1 TEASPOON FOR 70 KG PERSON (150 LB).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-229
Antidepressive Agents, Tricyclic
Substances that contain a fused three-ring moiety and are used in the treatment of depression. These drugs block the uptake of norepinephrine and serotonin into axon terminals and may block some subtypes of serotonin, adrenergic, and histamine receptors. However, the mechanism of their antidepressant effects is not clear because the therapeutic effects usually take weeks to develop and may reflect compensatory changes in the central nervous system. (See all compounds classified as Antidepressive Agents, Tricyclic.)
Histamine Antagonists
Drugs that bind to but do not activate histamine receptors, thereby blocking the actions of histamine or histamine agonists. Classical antihistaminics block the histamine H1 receptors only. (See all compounds classified as Histamine Antagonists.)
Sleep Aids, Pharmaceutical
Drugs used to induce SLEEP, prevent SLEEPLESSNESS, or treat SLEEP INITIATION AND MAINTENANCE DISORDERS. (See all compounds classified as Sleep Aids, Pharmaceutical.)
D - Dermatologicals
D04 - Antipruritics, incl. antihistamines, anesthetics, etc.
D04A - Antipruritics, incl. antihistamines, anesthetics, etc.
D04AX - Other antipruritics
D04AX01 - Doxepin
N - Nervous system
N06 - Psychoanaleptics
N06A - Antidepressants
N06AA - Non-selective monoamine reuptake inhibitors
N06AA12 - Doxepin
/DOXEPIN HAS/...A PECULIAR AFFINITY FOR UVEAL MELANIN...ALSO...BOUND BY OCULAR MELANIN BOTH IN VIVO & IN VITRO.
Grant, W. M. Toxicology of the Eye. 2nd ed. Springfield, Illinois: Charles C. Thomas, 1974., p. 427
AFTER HUMAN ORAL DOSE 75 MG DOXEPIN-HCL, EST 1ST-PASS METAB RANGED FROM 55-87% OF ORAL DOSE ASSUMING COMPLETE ABSORPTION.
ZIEGLER ET AL; CLIN PHARMACOL THER 23 (5) 573-9 (1978)
The pharmacokinetics of doxepin have not been extensively studied, but the drug is well absorbed from the GI tract in animals. Peak plasma concentrations occur within 2 hours after oral administration of the drug.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1807
Limited data indicate that doxepin and its active N-demethylated metabolite are distributed into milk in concentrations reportedly ranging from about 30-140% and 10-115%, respectively, of those in maternal serum and that substantial concentrations of the active metabolite have been detected in the serum and urine of nursing infants whose mothers were receiving 75-150 mg daily.
McEvoy, G.K. (ed.). American Hospital Formulary Service-Drug Information 19 98. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 1998 (Plus Supplements)., p. 1807
AFTER ORAL DOSING OF DOXEPIN-HCL TO HUMANS, METABOLITE DESMETHYLDOXEPIN WAS PRODUCED.
ZIEGLER ET AL; CLIN PHARMACOL THER 23 (5) 573-9 (1978)
Doxepin has known human metabolites that include Doxepin N-glucuronide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
AFTER ORAL 75 MG DOXEPIN-HCL TO HUMANS, PEAK PLASMA CONCN 8.8-45.8 NG/ML, REACHED WITHIN 4 HR. DISAPPEARANCE OF DOXEPIN WAS BIPHASIC & FOLLOWED 1ST-ORDER KINETICS. MEAN DOXEPIN T/2 WAS 16.8 HR. MEAN APPARENT VOL OF DISTRIBUTION WAS 20.2 L/KG.
ZIEGLER ET AL; CLIN PHARMACOL THER 23 (5) 573-9 (1978)
ACTION OF TRICYCLIC ANTIDEPRESSANTS ON CATECHOLAMINES & INDOLEAMINES IN BRAIN...BLOCK RE-UPTAKE OF NOREPINEPHRINE BY ADRENERGIC NERVE TERMINALS. /TRICYCLIC ANTIDEPRESSANTS/
Goodman, L.S., and A. Gilman. (eds.) The Pharmacological Basis of Therapeutics. 5th ed. New York: Macmillan Publishing Co., Inc., 1975., p. 176


