



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
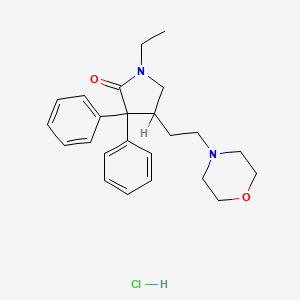

1. Docatone
2. Dopram
3. Doxapram
4. Hydrochloride, Doxapram
1. 113-07-5
2. Stimulexin
3. Dopram
4. 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenylpyrrolidin-2-one Monohydrochloride
5. Doxapram Hydrochloride Anhydrous
6. Doxapram (hydrochloride)
7. Fb8713u6dm
8. 1-ethyl-4-(2-morpholin-4-ylethyl)-3,3-diphenylpyrrolidin-2-one;hydrochloride
9. 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenylpyrrolidin-2-onemonohydrochloride
10. Dsstox_cid_27815
11. Dsstox_rid_82579
12. Dsstox_gsid_47837
13. Doxapramhydrochloride
14. Chebi:59837
15. Ahr-619
16. Cas-113-07-5
17. 3,3-diphenyl-1-ethyl-4-(2-morpholinoethyl)-2-pyrrolidinone Hydrochloride
18. 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone Hydrochloride Hydrate
19. 2-pyrrolidinone, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-, Monohydrochloride, Monohydrate
20. Ncgc00167459-01
21. Unii-fb8713u6dm
22. Einecs 204-022-3
23. 2-pyrrolidinone, 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-, Monohydrochloride
24. Schembl40645
25. Mls001424142
26. Chembl1200876
27. Dtxsid9047837
28. Tox21_112463
29. Mfcd00072148
30. Nsc170958
31. Akos015906444
32. Tox21_112463_1
33. Ac-4694
34. Ccg-101076
35. Ccg-221205
36. Nc00326
37. Nsc 170958
38. Sb18933
39. 2-pyrrolidinone, 1-ethyl-4-(2-(4-morpholinyl)ethyl)-3,3-diphenyl-, Monohydrochloride
40. Ncgc00167459-02
41. As-13336
42. Smr000469229
43. 2-pyrrolidinone,3-diphenyl-, Monohydrochloride
44. 081d530
45. 113d075
46. J-002892
47. Q27126918
48. 1-ethyl-4-(2-morpholinoethyl)-3,3-diphenylpyrrolidin-2-one Hydrochloride
49. (+/-)-1-ethyl-4-(2-morpholinoethyl)-3,3-diphenyl-2-pyrrolidinone Monohydrochloride
50. 2-pyrrolidinone, 1-ethyl-4-(2-(4-morpholinyl)ethyl)-3,3-diphenyl-, Hydrochloride, Hydrate (1:1:1)
51. 2-pyrrolidinone, 1-ethyl-4-(2-(4-morpholinyl)ethyl)-3,3-diphenyl-, Monohydrochloride, (+/-)-
| Molecular Weight | 415.0 g/mol |
|---|---|
| Molecular Formula | C24H31ClN2O2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 414.2074059 g/mol |
| Monoisotopic Mass | 414.2074059 g/mol |
| Topological Polar Surface Area | 32.8 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 487 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Doxapram hydrochloride |
| Drug Label | DOPRAM Injection (doxapram hydrochloride injection, USP) is a clear, colorless, sterile, non-pyrogenic, aqueous solution with pH 3.5 to 5, for intravenous administration.Each 1 mL contains:Doxapram Hydrochloride, USP .................................... |
| Active Ingredient | Doxapram hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml |
| Market Status | Prescription |
| Company | Bedford |
| 2 of 2 | |
|---|---|
| Drug Name | Doxapram hydrochloride |
| Drug Label | DOPRAM Injection (doxapram hydrochloride injection, USP) is a clear, colorless, sterile, non-pyrogenic, aqueous solution with pH 3.5 to 5, for intravenous administration.Each 1 mL contains:Doxapram Hydrochloride, USP .................................... |
| Active Ingredient | Doxapram hydrochloride |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 20mg/ml |
| Market Status | Prescription |
| Company | Bedford |
Central Nervous System Stimulants
A loosely defined group of drugs that tend to increase behavioral alertness, agitation, or excitation. They work by a variety of mechanisms, but usually not by direct excitation of neurons. The many drugs that have such actions as side effects to their main therapeutic use are not included here. (See all compounds classified as Central Nervous System Stimulants.)
Respiratory System Agents
Drugs used for their effects on the respiratory system. (See all compounds classified as Respiratory System Agents.)


