



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
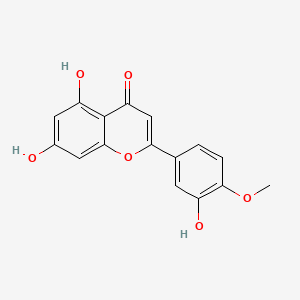

1. 520-34-3
2. Luteolin 4'-methyl Ether
3. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-chromen-4-one
4. 4'-methylluteolin
5. Salinigricoflavonol
6. Diosmetine
7. Luteolin 4-methyl Ether
8. 3',5,7-trihydroxy-4'-methoxyflavone
9. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one
10. 5,7,3'-trihydroxy-4'-methoxyflavone
11. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-benzopyrone
12. 4h-1-benzopyran-4-one, 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-
13. Mfcd00017425
14. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one
15. Chebi:4630
16. Twz37241ot
17. Flavone, 3',5,7-trihydroxy-4'-methoxy-
18. Pillon
19. Sr-05000002196
20. Unii-twz37241ot
21. Hsdb 8101
22. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4h-1-benzopyran-4-one (diosmetin)
23. Einecs 208-291-8
24. Diosmetol
25. Spectrum_001505
26. Specplus_000435
27. Diosmetin [mi]
28. Diosmetin [inci]
29. Spectrum2_001638
30. Spectrum3_000987
31. Spectrum4_001113
32. Spectrum5_001707
33. Diosmetin [who-dd]
34. Bspbio_002653
35. Kbiogr_001586
36. Kbioss_001985
37. Mls006011843
38. Chembl90568
39. Diosmetin, Analytical Standard
40. Divk1c_006531
41. Schembl289337
42. Spbio_001735
43. Bdbm23414
44. Kbio1_001475
45. Kbio2_001985
46. Kbio2_004553
47. Kbio2_007121
48. Kbio3_001873
49. Dtxsid80199966
50. Luteolin-4'-methyl Ether
51. 5,7-dihydroxy-2-(3-hydroxy-4-methoxy-phenyl)chromen-4-one
52. Hms3656g13
53. Bcp28283
54. Hy-n0125
55. Zinc5733652
56. Bbl027839
57. Ccg-38758
58. Diosmetin (luteolin 4-methyl Ether)
59. Lmpk12110824
60. S2380
61. Stl146390
62. Akos005720974
63. Cs-5455
64. Db11259
65. Sdccgmls-0066783.p001
66. 3',5,7-trihydroxy-4'-methoxy Flavone
67. Ncgc00163540-01
68. Ncgc00163540-02
69. Ncgc00163540-03
70. Ncgc00178549-01
71. Ac-34868
72. Bs-17174
73. Smr002044787
74. Sy066981
75. Cyanidenon-4'-methyl Ether 1479
76. Db-050154
77. D4441
78. Ft-0603442
79. Ft-0667618
80. Sw219332-1
81. C10038
82. 520d343
83. A828902
84. A871094
85. Q1649664
86. Sr-05000002196-2
87. Sr-05000002196-3
88. Brd-k26862302-001-02-9
89. Brd-k26862302-001-03-7
90. 2-(4-methoxy-3-oxidanyl-phenyl)-5,7-bis(oxidanyl)chromen-4-one
91. 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-1-benzopyran-4-one
92. J8d
| Molecular Weight | 300.26 g/mol |
|---|---|
| Molecular Formula | C16H12O6 |
| XLogP3 | 1.7 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 2 |
| Exact Mass | 300.06338810 g/mol |
| Monoisotopic Mass | 300.06338810 g/mol |
| Topological Polar Surface Area | 96.2 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 462 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Absorption
Diosmin is hydrolyzed to its aglycone diosmetin by intestinal microflora enzymes before its absorption into the body.
... Diosmetin was metabolised to the structurally similar flavone luteolin in MDA-MB 468 cells, whereas no metabolism was seen in MCF-10A cells...
PMID:19424633 Androutsopoulos VP et al; Oncol Rep 21(6):1525-8 (2009)
Various types of tumors are known to overexpress enzymes belonging to the CYP1 family of cytochromes P450. The present study aimed to characterize the metabolism and further antiproliferative activity of the natural flavonoid diosmetin in the CYP1-expressing human hepatoma cell line HepG2. Diosmetin was converted to luteolin in HepG2 cells after 12 and 30 hr of incubation. In the presence of the CYP1A inhibitor alpha-naphthoflavone, the conversion of diosmetin to luteolin was attenuated. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays revealed luteolin to be more cytotoxic than diosmetin. The antiproliferative effect of diosmetin in HepG2 cells was attributed to blockage at the G2/M phase as determined by flow cytometry. Induction of G2/M arrest was accompanied by up-regulation of phospho-extracellular-signal-regulated kinase (p-ERK), phospho-c-jun N-terminal kinase, p53 and p21 proteins. More importantly, induction of G2/M arrest and p53 and p-ERK up-regulation were reversed by the application of the CYP1 inhibitor alpha-naphthoflavone. Taken together, the data provide new evidence on the tumor-suppressing role of cytochrome P450 CYP1A enzymes and extend the hypothesis that the anticancer activity of dietary flavonoids is enhanced by P450-activation.
PMID:22749133 Androutsopoulos VP and Spandidos DA ; J Nutr Biochem 24(2):496-504 (2013)
CYP1A1 and CYP1B1 are two extrahepatic enzymes that have been implicated in carcinogenesis and cancer progression. Selective inhibition of CYP1A1 and CYP1B1 by dietary constituents, notably the class of flavonoids, is a widely accepted paradigm that supports the concept of dietary chemoprevention. In parallel, recent studies have documented the ability of CYP1 enzymes to selectively metabolize dietary flavonoids to conversion products that inhibit cancer cell proliferation. In the present study /the authors/ have examined the inhibition of CYP1A1 and CYP1B1-catalyzed EROD activity by 14 different flavonoids containing methoxy- and hydroxyl-group substitutions as well as the metabolism of the monomethoxylated CYP1-flavonoid inhibitor acacetin and the poly-methoxylated flavone eupatorin-5-methyl ether by recombinant CYP1A1 and CYP1B1. The most potent inhibitors of CYP1-EROD activity were the methoxylated flavones acacetin, diosmetin, eupatorin and the di-hydroxylated flavone chrysin, indicating that the 4'-OCH(3) group at the B ring and the 5,7-dihydroxy motif at the A ring play a prominent role in EROD inhibition. Potent inhibition of CYP1B1 EROD activity was also obtained for the poly-hydroxylated flavonols quercetin and myricetin. HPLC metabolism of acacetin by CYP1A1 and CYP1B1 revealed the formation of the structurally similar flavone apigenin by demethylation at the 4'-position of the B ring, whereas the flavone eupatorin-5-methyl ether was metabolized to an as yet unidentified metabolite assigned E(5)M1. Eupatorin-5-methyl ether demonstrated a submicromolar IC50 in the CYP1-expressing cancer cell line MDA-MB 468, while it was considerably inactive in the normal cell line MCF-10A. Homology modeling in conjunction with molecular docking calculations were employed in an effort to rationalize the activity of these flavonoids based on their CYP1-binding mode. Taken together the data suggest that dietary flavonoids exhibit three distinct modes of action with regard to cancer prevention, based on their hydroxyl and methoxy decoration: (1) inhibitors of CYP1 enzymatic activity, (2) CYP1 substrates and (3) substrates and inhibitors of CYP1 enzymes.
PMID:21482471 Androutsopoulos VP et al; Bioorg Med Chem 19(9):2842-9 (2011)
Flos Chrysanthemi (the flower of Chrysanthemum morifolium Ramat.) is widely used in China as a food and traditional Chinese medicine for many diseases. Luteolin and apigenin are two main bioactive components in Flos Chrysanthemi, and chrysoeriol and diosmetin are two methylated metabolites of luteolin in vivo by cathechol-O-methyltransferase (COMT). However, there was /a/ lack of pharmacokinetic information of chrysoeriol and diosmetin after oral administration of Flos Chrysanthemi extract (FCE). The present study aimed to develop an HPLC-UV method for simultaneous determination of rat plasma concentration of luteolin, apigenin, chrysoeriol and diosmetin and utilize it in pharmacokinetic study of the four compounds after orally giving FCE to rats. The method was successfully validated and applied to the pharmacokinetic study when oral administration of FCE to rats with or without co-giving a COMT inhibitor, entacapone. Chrysoeriol and diosmetin were detected in rat plasma after oral administration of FCE and their concentrations were significantly decreased after co-giving entacapone... In conclusion, a sensitive, accurate and reproducible HPLC-UV method for simultaneous determination of luteolin, apigenin, chrysoeriol and diosmetin in rat plasma were developed, pharmacokinetics of chrysoeriol and diosmetin combined with luteolin and apigenin were characterized after oral administration of FCE to rats, which gave us more information on pharmacokinetics and potential pharmacological effects of FCE in vivo.
PMID:22999990 Chen Z et al; Fitoterapia 83(8):1616-22 (2012)
Diosmetin has known human metabolites that include (2S,3S,4S,5R)-3,4,5-Trihydroxy-6-[5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxochromen-7-yl]oxyoxane-2-carboxylic acid.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Various types of tumors are known to overexpress enzymes belonging to the CYP1 family of cytochromes P450. The present study aimed to characterize the metabolism and further antiproliferative activity of the natural flavonoid diosmetin in the CYP1-expressing human hepatoma cell line HepG2. Diosmetin was converted to luteolin in HepG2 cells after 12 and 30 hr of incubation. In the presence of the CYP1A inhibitor alpha-naphthoflavone, the conversion of diosmetin to luteolin was attenuated. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays revealed luteolin to be more cytotoxic than diosmetin. The antiproliferative effect of diosmetin in HepG2 cells was attributed to blockage at the G2/M phase as determined by flow cytometry. Induction of G2/M arrest was accompanied by up-regulation of phospho-extracellular-signal-regulated kinase (p-ERK), phospho-c-jun N-terminal kinase, p53 and p21 proteins. More importantly, induction of G2/M arrest and p53 and p-ERK up-regulation were reversed by the application of the CYP1 inhibitor alpha-naphthoflavone. Taken together, the data provide new evidence on the tumor-suppressing role of cytochrome P450 CYP1A enzymes and extend the hypothesis that the anticancer activity of dietary flavonoids is enhanced by P450-activation.
PMID:22749133 Androutsopoulos VP and Spandidos DA ; J Nutr Biochem 24(2):496-504 (2013)
CYP1A1 and CYP1B1 are two extrahepatic enzymes that have been implicated in carcinogenesis and cancer progression. Selective inhibition of CYP1A1 and CYP1B1 by dietary constituents, notably the class of flavonoids, is a widely accepted paradigm that supports the concept of dietary chemoprevention. In parallel, recent studies have documented the ability of CYP1 enzymes to selectively metabolize dietary flavonoids to conversion products that inhibit cancer cell proliferation. In the present study /the authors/ have examined the inhibition of CYP1A1 and CYP1B1-catalyzed EROD activity by 14 different flavonoids containing methoxy- and hydroxyl-group substitutions as well as the metabolism of the monomethoxylated CYP1-flavonoid inhibitor acacetin and the poly-methoxylated flavone eupatorin-5-methyl ether by recombinant CYP1A1 and CYP1B1. The most potent inhibitors of CYP1-EROD activity were the methoxylated flavones acacetin, diosmetin, eupatorin and the di-hydroxylated flavone chrysin, indicating that the 4'-OCH(3) group at the B ring and the 5,7-dihydroxy motif at the A ring play a prominent role in EROD inhibition. Potent inhibition of CYP1B1 EROD activity was also obtained for the poly-hydroxylated flavonols quercetin and myricetin. HPLC metabolism of acacetin by CYP1A1 and CYP1B1 revealed the formation of the structurally similar flavone apigenin by demethylation at the 4'-position of the B ring, whereas the flavone eupatorin-5-methyl ether was metabolized to an as yet unidentified metabolite assigned E(5)M1. Eupatorin-5-methyl ether demonstrated a submicromolar IC50 in the CYP1-expressing cancer cell line MDA-MB 468, while it was considerably inactive in the normal cell line MCF-10A. Homology modeling in conjunction with molecular docking calculations were employed in an effort to rationalize the activity of these flavonoids based on their CYP1-binding mode. Taken together the data suggest that dietary flavonoids exhibit three distinct modes of action with regard to cancer prevention, based on their hydroxyl and methoxy decoration: (1) inhibitors of CYP1 enzymatic activity, (2) CYP1 substrates and (3) substrates and inhibitors of CYP1 enzymes.
PMID:21482471 Androutsopoulos VP et al; Bioorg Med Chem 19(9):2842-9 (2011)
The binding mechanism of molecular interaction between diosmetin and human serum albumin (HSA) in a pH 7.4 phosphate buffer was studied using atomic force microscopy (AFM) and various spectroscopic techniques including fluorescence, resonance light scattering (RLS), UV-vis absorption, circular dichroism (CD), and Fourier transform infrared (FT-IR) spectroscopy. Fluorescence data revealed that the fluorescence quenching of HSA by diosmetin was a static quenching procedure. The binding constants and number of binding sites were evaluated at different temperatures. The RLS spectra and AFM images showed that the dimension of the individual HSA molecules were larger after interaction with diosmetin. The thermodynamic parameters, /changes in enthalpy and entropy/ were calculated to be -24.56 kJ/mol and 14.67 J/mol/K, respectively, suggesting that the binding of diosmtin to HSA was driven mainly by hydrophobic interactions and hydrogen bonds. The displacement studies and denaturation experiments in the presence of urea indicated site I as the main binding site for diosmetin on HSA. The binding distance between diosmetin and HSA was determined to be 3.54 nm based on the Forster theory. Analysis of CD and FT-IR spectra demonstrated that HSA conformation was slightly altered in the presence of diosmetin.
PMID:22353148 Zhang G et al; J Agric Food Chem 60(10):2721-9 (2012)
The survival of osteoblasts is one of the determinants of the development of osteoporosis. This study /investigates/ the osteoblastic differentiation induced by diosmetin, a flavonoid derivative, in osteoblastic cell lines MG-63, hFOB, and MC3T3-E1 and bone marrow stroma cell line M2-10B4. Osteoblastic differentiation was determined by assaying alkaline phosphatase (ALP) activity and mineralization degree and measuring various osteoblast-related markers using ELISA. Expression and phosphorylation of Runt-related transcription factor 2 (Runx2), protein kinase Cdelta (PKCdelta), extracellular signal-regulated kinase (ERK), p38, and c-jun-N-terminal kinase (JNK) was assessed by immunoblot. Rac1 activity was determined by immunoprecipitation, and Runx2 activity was assessed by EMSA. Genetic inhibition was performed by small hairpin RNA plasmids or small interfering RNA (siRNA) transfection. Diosmetin exhibited an effect on osteoblastic maturation and differentiation by means of ALP activity, osteocalcin, osteopontin, and type I collagen production, as well as Runx2 upregulation. Induction of differentiation by diosmetin was associated with increased PKCdelta phosphorylation and the activations of Rac1 and p38 and ERK1/2 kinases. Blocking PKCdelta by siRNA inhibition significantly decreased osteoblastic differentiation by inhibiting Rac1 activation and subsequently attenuating the phosphorylation of p38 and ERK1/2. In addition, blocking p38 and ERK1/2 by siRNA transfection also suppressed diosmetin-induced cell differentiation. /This shows/ that diosmetin induced osteoblastic differentiation through the PKCdelta-Rac1-MEK3/6-p38 and PKCdelta-Rac1-MEK1/2- ERK1/2-Runx2 pathways and that it is a promising agent for treating osteoporosis.
PMID:18269307 Hsu YL and Kuo PL; J Bone Miner Res 23 (6): 949-60 (2008)


