
Synopsis
0
EU WC
0
KDMF
0
NDC API
0
Australia
0
South Africa
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Dimethyl Sulphoxide
2. Dimethylsulfoxide
3. Dimethylsulphinyl
4. Dimethylsulphoxide
5. Dimexide
6. Dmso
7. Rheumabene
8. Rimso
9. Rimso 100
10. Rimso 50
11. Rimso-50
12. Rimso50
13. Sclerosol
14. Sulfinylbis(methane)
15. Sulfoxide, Dimethyl
16. Sulphoxide, Dimethyl
1. Dmso
2. 67-68-5
3. Methyl Sulfoxide
4. Methylsulfinylmethane
5. Dimethylsulfoxide
6. Dimethyl Sulphoxide
7. Methane, Sulfinylbis-
8. Demsodrox
9. Demasorb
10. Demavet
11. Dimexide
12. Domoso
13. Dromisol
14. Durasorb
15. Infiltrina
16. Somipront
17. Syntexan
18. Deltan
19. Demeso
20. Dolicur
21. Hyadur
22. Sulfinylbismethane
23. Dimethyl Sulfur Oxide
24. Dermasorb
25. Dipirartril-tropico
26. Doligur
27. Kemsol
28. Gamasol 90
29. Sulfinylbis(methane)
30. Rimso-50
31. Topsym
32. Dimethylsulphoxide
33. Dimethylsulfoxid
34. Sq 9453
35. Dimethylsulfoxyde
36. Rimso 50
37. Nsc-763
38. Sulfinylbis-methane
39. Caswell No. 381
40. Dimetil Sulfoxido
41. Methyl Sulphoxide
42. Dimethyli Sulfoxidum
43. Ccris 943
44. (methylsulfinyl)methane
45. (ch3)2so
46. Dms-90
47. Nsc 763
48. A 10846
49. Dimethyl-sulfoxide
50. S(o)me2
51. M 176
52. Methane, 1,1'-sulfinylbis-
53. Epa Pesticide Chemical Code 000177
54. Dms 70
55. Dms 90
56. Ai3-26477
57. Dmso, Sterile Filtered
58. Mfcd00002089
59. Chembl504
60. Sq-9453
61. Nsc763
62. Yow8v9698h
63. Dimethyl Sulfoxide, Hplc Grade
64. Chebi:28262
65. Dimethyl Sulfoxide, 99%
66. 103759-08-6
67. Topsym (rescinded)
68. Rimso-5
69. Domoso (veterinary)
70. Methylsulfoxide
71. Dimexidum
72. Sulfinyldimethane
73. Dimetilsolfossido
74. Dimetilsolfossido [dcit]
75. Dimethyl Sulpoxide
76. Fatty Acids, Tall-oil, Polymers With Me Epoxyoctadecanoate And Tetraethylenepentamine
77. Hsdb 80
78. Sulfoxide, Dimethyl
79. Methanesulfinylmethane
80. Dms-70
81. Dimethylsulfoxyde [inn-french]
82. Dimetil Sulfoxido [inn-spanish]
83. (methanesulfinyl)methane
84. Dimethyli Sulfoxidum [inn-latin]
85. Einecs 200-664-3
86. C2h6os
87. Unii-yow8v9698h
88. Diluent
89. Dimethysulfoxide
90. Dimethlysulfoxide
91. Dimethvlsulfoxide
92. Dimethyisulfoxide
93. Dimethylsulphoxid
94. Dimethy Sulfoxide
95. Dimetyl Sulfoxide
96. Dimethyisulphoxide
97. Dtxsid2021735
98. Dimethyl Sulfoxyde
99. Dimethyl-sulfoxyde
100. Dimethyl Suiphoxide
101. Dimethyl-sulphoxide
102. Dirnethyl Sulfoxide
103. Dimethyl Sulfoxixde
104. Methylsulfmylmethane
105. Dimethyl Sulf Oxide
106. Sulfinyl Bis(methane)
107. 2-thiapropane2-oxide
108. Dimethyl Sulfoxide [usan:usp:inn:ban]
109. Dimethylsulfoxide Solution
110. Methyl Sulfoxide (8ci)
111. Rimso-50 (tn)
112. Dimethyl Sulfoxide (dmso)
113. Dimethyl Sulfoxide(dmso)
114. Dmso (sterile-filtered)
115. Dmso [inci]
116. Dmso, Dimethyl Sulfoxide
117. Dsstox_cid_1735
118. Dimethyl Sulfoxide Solution
119. (dmso)
120. Dmso (dimethyl Sulfoxide)
121. Ec 200-664-3
122. Dsstox_rid_76298
123. H3c-so-ch3
124. Bidd:pxr0182
125. Dsstox_gsid_21735
126. Dimethyl Sulfoxide, >=99%
127. Dimethyl Sulfoxide, Anhydrous
128. Dimethyl Sulfoxide, For Hplc
129. Methane, Sulfinylbis- (9ci)
130. Wln: Os1&1
131. Dimethyl Sulfoxide [ii]
132. Dimethyl Sulfoxide [mi]
133. Dimethyl Sulfoxide [inn]
134. Dimethyl Sulfoxide [jan]
135. Dimethyl Sulfoxide, >=99.5%
136. Dimethyl Sulfoxide, Pcr Reagent
137. Dimethyl Sulfoxide [hsdb]
138. Dimethyl Sulfoxide [usan]
139. Dimethyl Sulfoxide, Acs Reagent
140. Methyl Sulfoxide, >=99%, Fg
141. Dimethyl Sulfoxide, P.a., 99%
142. Dimethyl Sulfoxide [vandf]
143. Dimethyl Sulfoxide, Lr, >=99%
144. Pharmakon1600-01506122
145. Dimethyl Sulfoxide [mart.]
146. Amy14894
147. Cs-b1637
148. Dimethyl Sulfoxide (jan/usp/inn)
149. Dimethyl Sulfoxide [usp-rs]
150. Dimethyl Sulfoxide [who-dd]
151. Hy-y0320
152. Methylsulfinylmethane [fhfi]
153. Zinc5224188
154. Tox21_300957
155. Bdbm50026472
156. Nsc760436
157. Stl264194
158. Dimethyl Sulfoxide, Ar, >=99.5%
159. Akos000121107
160. Ccg-213615
161. Db01093
162. Dimethyl Sulfoxide [green Book]
163. Dimethyl Sulfoxide, Analytical Standard
164. Nsc-760436
165. Cas-67-68-5
166. Dimethyl Sulfoxide [orange Book]
167. Mrf-0000764
168. (methanesulfinyl)methanedimethyl Sulfoxide
169. Dimethyl Sulfoxide [ep Monograph]
170. Dimethyl Sulfoxide, For Molecular Biology
171. Ncgc00163958-01
172. Ncgc00163958-02
173. Ncgc00163958-03
174. Ncgc00254859-01
175. Dimethyl Sulfoxide [usp Monograph]
176. Dimethyl Sulfoxide, Anhydrous, >=99.9%
177. Dimethyl Sulfoxide, Hplc Grade, 99.9%
178. Dimethyl Sulfoxide [for Spectrophotometry]
179. Dimethyl Sulfoxide, For Hplc, >=99.5%
180. Dimethyl Sulfoxide, For Hplc, >=99.7%
181. Ds-015031
182. D0798
183. D1159
184. D5293
185. Dimethyl Sulfoxide, Acs Reagent, >=99.9%
186. Dimethyl Sulfoxide, Aldrasorb(tm), 99.8%
187. Ft-0625099
188. Ft-0625100
189. D01043
190. Dimethyl Sulfoxide 1000 Microg/ml In Methanol
191. Dimethyl Sulfoxide Solution, 50 Wt. % In H2o
192. Dimethyl Sulfoxide, >=99.6%, Reagentplus(r)
193. Dimethyl Sulfoxide, Reagentplus(r), >=99.5%
194. Ab01563146_01
195. Dimethyl Sulfoxide (dmso), Cell Culture Reagent
196. Dimethyl Sulfoxide, P.a., Acs Reagent, 99.9%
197. Dimethyl Sulfoxide, Saj First Grade, >=99.0%
198. Dimethyl Sulfoxide 100 Microg/ml In Acetonitrile
199. Dimethyl Sulfoxide, Jis Special Grade, >=99.0%
200. Dimethyl Sulfoxide, Vetec(tm) Reagent Grade, 99%
201. Q407927
202. Dimethyl Sulfoxide, Uv Hplc Spectroscopic, 99.9%
203. Dimethyl Sulfoxide, Anhydrous, Zero2(tm), >=99.9%
204. Dimethyl Sulfoxide, Meets Ep, Usp Testing Specifications
205. Dimethyl Sulfoxide, Acs Spectrophotometric Grade, >=99.9%
206. Dimethyl Sulfoxide, Puriss. P.a., Dried, <=0.02% Water
207. 4h-1,3-oxazine,2-cyclopentyl-5,6-dihydro-4,4,7-trimethyl-
208. Dimethyl Sulfoxide, >=99.5% (gc), Plant Cell Culture Tested
209. Dimethyl Sulfoxide, Bioultra, For Molecular Biology, >=99.5% (gc)
210. Dimethyl Sulfoxide, European Pharmacopoeia (ep) Reference Standard
211. Dimethyl Sulfoxide, Puriss. P.a., Acs Reagent, >=99.9% (gc)
212. Dimethyl Sulfoxide, Vetec(tm) Reagent Grade, Anhydrous, >=99.7%
213. Dimethyl Sulfoxide, >=99.0%, Suitable For Absorption Spectrum Analysis
214. Dimethyl Sulfoxide, United States Pharmacopeia (usp) Reference Standard
215. Dimethyl Sulfoxide, For Inorganic Trace Analysis, >=99.99995% (metals Basis)
216. Dimethyl Sulfoxide, Meets Ep Testing Specifications, Meets Usp Testing Specifications
217. Dimethyl Sulfoxide, Hybri-max(tm), Sterile-filtered, Bioreagent, Suitable For Hybridoma, >=99.7%
218. Dimethyl Sulfoxide, Puriss., Absolute, Over Molecular Sieve (h2o <=0.005%), >=99.5% (gc)
219. Dimethyl Sulfoxide, Sterile-filtered, Bioperformance Certified, Meets Ep, Usp Testing Specifications, Suitable For Hybridoma
| Molecular Weight | 78.14 g/mol |
|---|---|
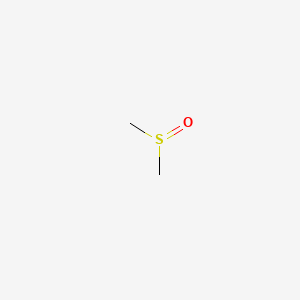
| Molecular Formula | C2H6OS |
| XLogP3 | -0.6 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 78.01393598 g/mol |
| Monoisotopic Mass | 78.01393598 g/mol |
| Topological Polar Surface Area | 36.3 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 29 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 6 | |
|---|---|
| Drug Name | Dimethyl sulfoxide |
| PubMed Health | Dimethyl Sulfoxide (Inside the bladder) |
| Drug Classes | Renal-Urologic Agent |
| Drug Label | RIMSO-50(dimethyl sulfoxide) (DMSO) 50% w/w Aqueous Solution for intravesical instillation.Each mL contains 0.54 gm dimethyl sulfoxide STERILE AND NON-PYROGENIC.Intravesical instillation for the treatment of interstitial cystitis.NOT FOR INTRAMUSCU... |
| Active Ingredient | Dimethyl sulfoxide |
| Dosage Form | Solution |
| Route | Intravesical |
| Strength | 50% |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 2 of 6 | |
|---|---|
| Drug Name | Rimso-50 |
| PubMed Health | Dimethyl Sulfoxide (Inside the bladder) |
| Drug Classes | Renal-Urologic Agent |
| Drug Label | RIMSO-50(dimethyl sulfoxide) (DMSO) 50% w/w Aqueous Solution for intravesical instillation.Each mL contains 0.54 gm dimethyl sulfoxide STERILE AND NON-PYROGENIC.Intravesical instillation for the treatment of interstitial cystitis.NOT FOR INTRAMUSCU... |
| Active Ingredient | Dimethyl sulfoxide |
| Dosage Form | Solution |
| Route | Intravesical |
| Strength | 50% |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 3 of 6 | |
|---|---|
| Drug Name | Sclerosol |
| PubMed Health | Talc (Intrapleural) |
| Drug Classes | Sclerosing Agent |
| Active Ingredient | Talc |
| Dosage Form | Aerosol, metered |
| Route | Intrapleural |
| Strength | 400mg/spray |
| Market Status | Prescription |
| Company | Bryan |
| 4 of 6 | |
|---|---|
| Drug Name | Dimethyl sulfoxide |
| PubMed Health | Dimethyl Sulfoxide (Inside the bladder) |
| Drug Classes | Renal-Urologic Agent |
| Drug Label | RIMSO-50(dimethyl sulfoxide) (DMSO) 50% w/w Aqueous Solution for intravesical instillation.Each mL contains 0.54 gm dimethyl sulfoxide STERILE AND NON-PYROGENIC.Intravesical instillation for the treatment of interstitial cystitis.NOT FOR INTRAMUSCU... |
| Active Ingredient | Dimethyl sulfoxide |
| Dosage Form | Solution |
| Route | Intravesical |
| Strength | 50% |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 5 of 6 | |
|---|---|
| Drug Name | Rimso-50 |
| PubMed Health | Dimethyl Sulfoxide (Inside the bladder) |
| Drug Classes | Renal-Urologic Agent |
| Drug Label | RIMSO-50(dimethyl sulfoxide) (DMSO) 50% w/w Aqueous Solution for intravesical instillation.Each mL contains 0.54 gm dimethyl sulfoxide STERILE AND NON-PYROGENIC.Intravesical instillation for the treatment of interstitial cystitis.NOT FOR INTRAMUSCU... |
| Active Ingredient | Dimethyl sulfoxide |
| Dosage Form | Solution |
| Route | Intravesical |
| Strength | 50% |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 6 of 6 | |
|---|---|
| Drug Name | Sclerosol |
| PubMed Health | Talc (Intrapleural) |
| Drug Classes | Sclerosing Agent |
| Active Ingredient | Talc |
| Dosage Form | Aerosol, metered |
| Route | Intrapleural |
| Strength | 400mg/spray |
| Market Status | Prescription |
| Company | Bryan |
Cryoprotective Agents; Free Radical Scavengers; Solvents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2014); Available from, as of August 1, 2014: https://www.nlm.nih.gov/cgi/mesh/2014/MB_cgi?term=Dimethyl%20sulfoxide
DMSO may have anti-inflammatory, antioxidant and analgesic activities. DMSO also readily penetrates cellular membranes.
PDR for Nutritional Supplements 2nd ed. Thomson Reuters, Montvale, NJ 2008, p. 187
EXPL THER OBJECTIVE: To evaluate the discomfort and long-term efficacy associated with instillation of dimethyl sulfoxide (DMSO). MATERIAL AND METHODS: A total of 28 patients, 13 (11 females, 2 males) with classic interstitial cystitis (IC) and 15 (13 females, two males) with non-ulcer disease, who had received at least one series of six instillations of DMSO were studied. In addition to studying micturition diaries before and after the treatment, the evaluation included assessments of pain using a visual analog scale and of side-effects after each instillation in every series. Data were obtained by surveying the clinical records. A follow-up telephone interview was conducted for those patients who were treated with DMSO and in whom the treatment was considered successful. DMSO instillations were considered successful if the patient reported symptom amelioration and chose to continue with the treatment. RESULTS: Side-effects were not more common or pronounced in patients with classic compared to non-ulcer IC. For classic IC a significant difference could be seen when comparing side-effects experienced during the first three instillations and the three subsequent instillations. After DMSO instillations, a residual treatment effect lasting 16-72 months could be seen. CONCLUSIONS: Intravesical instillation therapy with DMSO appears to be a feasible treatment option for both subtypes of IC and is associated with a reasonably low degree of discomfort.
PMID:15764276 Rossberger J et al; Scand J Urol Nephrol. 39(1):73-7 (2005).
/Dimethyl sulfoxide is indicated/ for the symptomatic relief of patients with interstitial cystitis. /Included in US product labeling/
Drug Facts and Comparisons 2013. Wolters Kluwer Health St. Louis, MO 2013, p. 1067
For more Therapeutic Uses (Complete) data for DIMETHYL SULFOXIDE (12 total), please visit the HSDB record page.
Onyx injection is a new technique for embolization of cerebral aneurysms that is involved in a controversy about the 'toxicity' of its solvent, dimethyl sulfoxide (DMSO). /The study/ retrospectively studied 38 patients treated for aneurysms with the liquid polymer, Onyx. Induction was with propofol, fentanyl and vecuronium, and anesthesia was maintained with isoflurane in O2 and N2O. The patients were given 500 mL of fluid after induction, and bradycardia was prevented in order to keep patients hyperdynamic. Electrocardiography (ECG), non-invasive blood pressure (NIBP), pulse oximetry, core temperatures, invasive blood pressure (BP), etCO2, and urine output were monitored throughout the intervention. Heart rate and BP changes in response to balloon inflation, DMSO injection, Onyx injection and balloon deflation were recorded. The patients were followed with serial neurological examinations, computerized tomography and/or magnetic resonance imaging postoperatively for evidence of any neurological injury. Cumulative DMSO doses were always well under previously implicated doses for systemic toxicity. No changes implicating toxic reactions were observed during DMSO and Onyx injections. Balloon-induced changes returned to baseline within 1 min of balloon deflation. Technique-related permanent morbidity occurred in two patients (worsening of cranial nerve palsies in one and monocular blindness in another) and intracranial hemorrhage with resulting death in one patient. All patients showed a tendency to oxygen desaturation, but this finding did not cause any clinical consequence. Anesthesiologists need to be vigilant in monitoring patients treated with techniques that are new or are being developed. /The study/ have seen no evidence of toxicity or any anesthetic complications in our group of patients, our only clinical concern being a tendency to oxygen desaturation, which may be explained by the inhalational elimination of DMSO.
PMID:15868171 Pamuk AG et al; Neuroradiology. 2005 May;47(5):380-6 (2005).
/This study/ describe/s/ the occurrence of the trigeminocardiac reflex (TCR) during DMSO pre-flushing of the microcatheter in preparation for Onyx embolization via the internal maxillary artery. TCR has not been previously associated with embolization of extradural entities. Familiarity with this clinical reflex and its proper management may help in planning neurointerventional procedures involving DMSO injection in the trigeminal territory.
PMID:21561553 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3278028 Puri AS et al; Interv Neuroradiol. 17(1):13-6 (2011).
Stem cell transplants are established therapy for hematologic and solid tumor malignancies. Known neurological complications of stem cell transplantation include CNS infection, seizures, strokes, metabolic encephalopathy, and hemorrhage. /This paper/ report/s/ two cases of autologous stem cell transplantation complicated by cerebral infarction and myocardial injury. /It is postulated/ that the cryopreservative dimethyl sulfoxide may be responsible.
PMID:17353475 Chen-Plotkin AS et al; Neurology. 68(11):859-61 (2007).
It is not known whether this drug is excreted in human milk ... caution should be exercised when dimethyl sulfoxide is administered to a nursing woman.
Drug Facts and Comparisons 2013. Wolters Kluwer Health St. Louis, MO 2013, p. 1068
For more Drug Warnings (Complete) data for DIMETHYL SULFOXIDE (20 total), please visit the HSDB record page.
For the symptomatic relief of patients with interstitial cystitis.
Dimethyl Sulfoxide may have anti-inflammatory, antioxidant and analgesic activities. Dimethyl Sulfoxide also readily penetrates cellular membranes. The membrane-penetrating ability of dimethyl sulfoxide may enhance diffusion of other substances through the skin. For this reason, mixtures of idoxuridine and dimethyl sulfoxide have been used for topical treatment of herpes zoster in the United Kingdom.
Cryoprotective Agents
Substances that provide protection against the harmful effects of freezing temperatures. (See all compounds classified as Cryoprotective Agents.)
Solvents
Liquids that dissolve other substances (solutes), generally solids, without any change in chemical composition, as, water containing sugar. (Grant and Hackh's Chemical Dictionary, 5th ed) (See all compounds classified as Solvents.)
Free Radical Scavengers
Substances that eliminate free radicals. Among other effects, they protect PANCREATIC ISLETS against damage by CYTOKINES and prevent myocardial and pulmonary REPERFUSION INJURY. (See all compounds classified as Free Radical Scavengers.)
G - Genito urinary system and sex hormones
G04 - Urologicals
G04B - Urologicals
G04BX - Other urologicals
G04BX13 - Dimethyl sulfoxide
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AX - Other topical products for joint and muscular pain
M02AX03 - Dimethyl sulfoxide
Absorption
Readily and rapidly absorbed following administration by all routes and distributed throughout the body.
Route of Elimination
Dimethyl sulfoxide and dimethyl sulfone are excreted in the urine and feces.
Following topical application, DMSO is absorbed and widely distributed in tissue and body fluids. DMSO and dimethyl sulfone are excreted in the urine and feces. DMSO is eliminated through the breath and skin and is responsible for the characteristic garlic odor. ... Dimethyl sulfone can persist in serum > 2 weeks after a single intravesical instillation. No residual accumulation of DMSO has occurred after treatment from protracted periods of time.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 720
Dimethyl sulfoxide and /one of its metabolites/ dimethyl sulfone, are excreted in the urine and feces. Dimethyl sulfide /another metabolite/ is eliminated through the breath and skin...
Drug Facts and Comparisons 2013. Wolters Kluwer Health St. Louis, MO 2013, p. 1067
By use of a Fourier transform infrared (FTIR) spectroscopic imaging technique, /this study examined/ the dynamic optical clearing processes occurring in hyperosmotically biocompatible agents penetrating into skin tissue in vitro. The sequential collection of images in a time series provides an opportunity to assess penetration kinetics of dimethyl sulphoxide (DMSO) and glycerol beneath the surface of skin tissue over time. From 2-D IR spectroscopic images and 3-D false color diagrams, ...show/s/ that glycerol takes at least 30 min to finally penetrate the layer of epidermis, while DMSO can be detected in epidermis after only 4 min of being topically applied over stratum corneum sides of porcine skin. The results demonstrate the potential of a FTIR spectroscopic imaging technique as an analytical tool for the study of dynamic optical clearing effects when the bio-tissue is impregnated by hyperosmotically biocompatible agents such as glycerol and DMSO.
PMID:18465954 Jiang J et al; J Biomed Opt. 13(2):021105 (2008).
In man radioactivity of 35S DMSO appeared in blood 5 min after cutaneous application. One hour later, radioactivity could detected in bones.
EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. Dimethyl sulfoxide. Available from, as of June 7, 2005: https://www.epa.gov/hpv/pubs/hpvrstp.htm
For more Absorption, Distribution and Excretion (Complete) data for DIMETHYL SULFOXIDE (9 total), please visit the HSDB record page.
Dimethyl sulfoxide is metabolized in man by oxidation to dimethyl sulfone or by reduction in dimethyl sulfide. Dimethyl sulfoxide and dimethyl sulfone are excreted in the urine and feces.
Dimethyl sulfoxide is metabolized in man by oxidation to dimethyl sulfone or by reduction to dimethyl sulfide.
Drug Facts and Comparisons 2013. Wolters Kluwer Health St. Louis, MO 2013, p. 1067
Autoimmune strain MRL/Ipr, C3H/lpr, and male BXSB mice were placed on a continuous treatment regimen with 3% DMSO or 3% DMS02 in the drinking water, ad libitum, commencing at 1 to 2 months of age, before spontaneous autoimmune lymphoproliferative disease development could be detected. This represented doses of 8-10 g/kg/day of DMSO and 6-8 g/kg/day of DMS02. Both compounds were observed to extend the mean life span of MRL/Ipr mice from 5.5 months to over 10 months of age. All strains showed decreased antinuclear antibody responses and significant diminution of lymphadenopathy, splenomegaly, and anemia development. Serum IgG levels and spleen IgM antibody plaque formation, however, did not differ from control values. There was no indication of involvement of systemic immunosuppressive or antiproliferative effects, and treated animals were observed to remain healthy and vigorous with no signs of toxicity. These results demonstrate that high doses of both DMSO and its major in vivo metabolite, DMSO2, provide significant protection against the development of murine autoimmune lymphoproliferative disease.
EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. Dimethyl sulfoxide. Available from, as of June 7, 2005: https://www.epa.gov/hpv/pubs/hpvrstp.htm
In man, DMSO is oxidized into dimethylsulfone DMSO2, metabolite excreted by urine (17-22 %). DMSO is reduced into dimethylsulfide, DMS, a volatile metabolite, responsible for garlic odour of exhaled air (1 %). About 85 % is excreted unchanged, both by urine (50 %) and feces (50 %).
EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. Dimethyl sulfoxide. Available from, as of June 7, 2005: https://www.epa.gov/hpv/pubs/hpvrstp.htm
Unchanged DMSO has a half-life of 12 to 15 hours.
Novak, K.M. (ed.). Drug Facts and Comparisons 59th Edition 2005. Wolters Kluwer Health. St. Louis, Missouri 2005., p. 720
/The/ half-life /of DMSO in the rhesus monkey/ was calculated to be about 38 hrs and its elimination rate constant equaled 0.018, or about 2% per hr.
EPA/Office of Pollution Prevention and Toxics; High Production Volume (HPV) Challenge Program's Robust Summaries and Test Plans. Dimethyl sulfoxide. Available from, as of June 7, 2005: https://www.epa.gov/hpv/pubs/hpvrstp.htm
The mechanism of dimethyl sulfoxide's actions is not well understood. Dimethyl sulfoxide has demonstrated antioxidant activity in certain biological settings. For example, the cardiovascular protective effect of dimethyl sulfoxide in copper-deficient rats is thought to occur by an antioxidant mechanism. It is also thought that dimethyl sulfoxide's possible anti-inflammatory activity is due to antioxidant action.
Thioacetamide (400 mg/kg body weight, i.p.) was administered to rats. After 12 hr the activity of plasma glutamate-oxaloacetate transaminase (GOT) and glutamate-pyruvate transaminase (GPT) was significantly higher than that of the control group, and after 24 hr plasma GOT and GPT activities strongly increased. These results indicated that the necrotic process was initiated at about 12 hr and developed thereafter. By co-administration of dimethyl sulphoxide (DMSO, 18 and 1 hr before, and 8 hr after administration of thioacetamide: each time, 2.5 mL/kg body weight, p.o.), plasma GOT and GPT were significantly decreased and were even comparable to the control group, showing that DMSO totally prevented the necrotic action of thioacetamide. After 12 and 24 hr of thioacetamide administration, the hepatic level of vitamin C, the most sensitive chemical indicator of oxidative stress, decreased significantly, indicating that oxidative stress was significantly enhanced 12 hr after thioacetamide intoxication and thereafter. DMSO totally restored the liver vitamin C level, demonstrating that DMSO effectively ameliorated the oxidative stress caused by thioacetamide, resulting in the prevention of necrosis of the liver. Phosphorylated c-Jun NH(2)-terminal kinase (JNK) significantly increased transiently 12 hr after treatment with thioacetamide. These results indicated that oxidative stress and the activation of JNK took place almost simultaneously. Phosphorylated extracellular signal-related kinase (ERK) 2 was significantly increased 6-12 hr after thioacetamide injection. Phosphorylated p38 MAPK (mitogen activated protein kinase) was significantly decreased 24 hr after administration of thioacetamide. DMSO treatment inhibited the change of these MAPKs by thioacetamide, corresponding with the prevention of the liver necrosis as well as the attenuation of oxidative stress.
PMID:17395177 Kishioka T et al; Eur J Pharmacol 564 (1-3): 190-5 (2007)
Previous studies performed in our laboratory indicated that non-toxic concentrations of peroxynitrite nevertheless commit U937 cells to a rapid necrosis that is however prevented by a survival signaling driven by cytosolic phospholipase A(2)-released arachidonic acid. Toxicity was mediated by concentrations of peroxynitrite resulting in H(2)O(2)-dependent inhibition of arachidonic acid release. The present study shows that U937 cells differentiated to monocytes by prolonged exposure to dimethyl sulfoxide are resistant to peroxynitrite because able to respond with enhanced release of arachidonic acid. An additional important observation was that these cells require more arachidonate than the undifferentiated cells to support the survival signaling. The enhanced arachidonic acid release was not associated with changes in cytosolic phospholipase A(2) expression but was rather dependent on the increased responsiveness of the enzyme to calcium-dependent stimulation as well as on reduced mitochondrial formation of H(2)O(2). The latter event was found to be critical, since differentiated and undifferentiated cells were equally sensitive to peroxynitrite when the accumulation of H(2)O(2) was enhanced via depletion of catalase, or addition of a complex III inhibitor. Thus, the strategy selected by the differentiation process to allow monocytes to cope with peroxynitrite appears to involve some specific mechanism preventing the mitochondrial formation of H(2)O(2).
PMID:16103003 Guidarelli A et al; Int J Biochem Cell Biol. 38(1):56-68 (2006).
Dimethyl sulfoxide (DMSO) has recently been proposed as an anti-inflammatory and free radical scavenging agent. However, the mechanisms by which DMSO mediates its therapeutic effects are unclear. /This paper/ investigated the capability of DMSO to up-regulate heme oxygenase-1(HO-1) expression, as well as the possible underlying mechanisms in human umbilical vein endothelial cells (HUVECs). DMSO induced HO-1 expression both at the level of mRNA and protein in dose-and time-dependent manners in HUVECs, resulting in increased HO-1 activity. The pharmacological inhibition of cJun-N-terminal kinases (JNKs) blocked the DMSO-induced HO-1 up-regulation, while inhibition of extracellular regulated kinase and p38-MAPK did not block heme oxygenase-1 up-regulation. In addition, the phosphorylation of JNKs was initiated by DMSO, indicating the involvement of this kinase in the observed response. DMSO increased the nuclear translocation of NF-E2-related factor 2 (Nrf2) and enhanced its binding to the anti-oxidant response element. Inhibition of Nrf2 synthesis by small interfering RNA molecules subsequently inhibited HO-1 expression induced by DMSO, indicating DMSO's role in inducing HO-1 expression via Nrf2 activation. Utilizing these findings, the present study identified DMSO as a novel inducer of HO-1 expression and identified the underlying mechanisms involved in this process.
PMID:21533649 Liang C et al; Mol Cell Biochem. 355(1-2):109-15 (2011).
Dimethyl sulfoxide (DMSO) is evident to induce apoptosis in certain tumor cells in vitro. However, its apoptotic mechanism remains unexplored in in vivo tumors. This article describes that DMSO, being non-toxic to the normal lymphocytes, up regulated TNFalpha and p53, declined Bcl-2/Bax ratio, activated caspase 9 and PARP-1 cleavage and produced apoptotic pattern of DNA ladder in Dalton's lymphoma (DL) in vivo. This was consistent with the declined expressions of tumor growth supportive glycolytic enzymes; inducible D-fructose-6-phosphate-2-kinase and lactate dehydrogenase-5 in the DL cells. The findings suggest induction of TNFalpha-p53-mitochondrial pathway of apoptosis by DMSO in a non-Hodgkin's lymphoma and support evolving concept of glycolytic inhibition led apoptosis in a tumor cell in vivo.
PMID:21269693 Koiri RK and Trigun SK. Leuk Res. 35(7):950-6 (2011).

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
45
PharmaCompass offers a list of Dimethyl Sulfoxide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dimethyl Sulfoxide manufacturer or Dimethyl Sulfoxide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dimethyl Sulfoxide manufacturer or Dimethyl Sulfoxide supplier.
PharmaCompass also assists you with knowing the Dimethyl Sulfoxide API Price utilized in the formulation of products. Dimethyl Sulfoxide API Price is not always fixed or binding as the Dimethyl Sulfoxide Price is obtained through a variety of data sources. The Dimethyl Sulfoxide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dimethyl Sulfoxide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dimethyl Sulfoxide, including repackagers and relabelers. The FDA regulates Dimethyl Sulfoxide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dimethyl Sulfoxide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dimethyl Sulfoxide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dimethyl Sulfoxide supplier is an individual or a company that provides Dimethyl Sulfoxide active pharmaceutical ingredient (API) or Dimethyl Sulfoxide finished formulations upon request. The Dimethyl Sulfoxide suppliers may include Dimethyl Sulfoxide API manufacturers, exporters, distributors and traders.
click here to find a list of Dimethyl Sulfoxide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dimethyl Sulfoxide DMF (Drug Master File) is a document detailing the whole manufacturing process of Dimethyl Sulfoxide active pharmaceutical ingredient (API) in detail. Different forms of Dimethyl Sulfoxide DMFs exist exist since differing nations have different regulations, such as Dimethyl Sulfoxide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dimethyl Sulfoxide DMF submitted to regulatory agencies in the US is known as a USDMF. Dimethyl Sulfoxide USDMF includes data on Dimethyl Sulfoxide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dimethyl Sulfoxide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dimethyl Sulfoxide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The Dimethyl Sulfoxide Drug Master File in Japan (Dimethyl Sulfoxide JDMF) empowers Dimethyl Sulfoxide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the Dimethyl Sulfoxide JDMF during the approval evaluation for pharmaceutical products. At the time of Dimethyl Sulfoxide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of Dimethyl Sulfoxide suppliers with JDMF on PharmaCompass.
A Dimethyl Sulfoxide CEP of the European Pharmacopoeia monograph is often referred to as a Dimethyl Sulfoxide Certificate of Suitability (COS). The purpose of a Dimethyl Sulfoxide CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Dimethyl Sulfoxide EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Dimethyl Sulfoxide to their clients by showing that a Dimethyl Sulfoxide CEP has been issued for it. The manufacturer submits a Dimethyl Sulfoxide CEP (COS) as part of the market authorization procedure, and it takes on the role of a Dimethyl Sulfoxide CEP holder for the record. Additionally, the data presented in the Dimethyl Sulfoxide CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Dimethyl Sulfoxide DMF.
A Dimethyl Sulfoxide CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Dimethyl Sulfoxide CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Dimethyl Sulfoxide suppliers with CEP (COS) on PharmaCompass.
Dimethyl Sulfoxide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dimethyl Sulfoxide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dimethyl Sulfoxide GMP manufacturer or Dimethyl Sulfoxide GMP API supplier for your needs.
A Dimethyl Sulfoxide CoA (Certificate of Analysis) is a formal document that attests to Dimethyl Sulfoxide's compliance with Dimethyl Sulfoxide specifications and serves as a tool for batch-level quality control.
Dimethyl Sulfoxide CoA mostly includes findings from lab analyses of a specific batch. For each Dimethyl Sulfoxide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dimethyl Sulfoxide may be tested according to a variety of international standards, such as European Pharmacopoeia (Dimethyl Sulfoxide EP), Dimethyl Sulfoxide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dimethyl Sulfoxide USP).

