



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
0
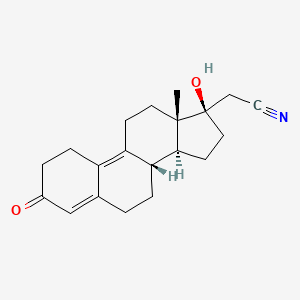

1. 17 Alpha-cyanomethyl-17 Beta-hydroxy-13 Beta-methylgona-4,9-dien-3-one
2. 17 Alpha-cyanomethyl-17 Beta-hydroxyestra-4,9(10)-diene-3-one
3. 19-norpregna-4,9-diene-21-nitrile, 17-hydroxy-3-oxo-, (17alpha)-
4. Sts 557
5. Sts-557
1. 65928-58-7
2. Dienogestrel
3. Dienogestril
4. Sts 557
5. Endometrion
6. Dienogestum
7. Dinagest
8. Natazia
9. Sts-557
10. Visanne
11. Mjr-35
12. 17alpha-17-hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile
13. Bay86-5258
14. Bay 86-5258
15. 46m3ev8hhe
16. Zk 37659
17. 2-[(8s,13s,14s,17r)-17-hydroxy-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]acetonitrile
18. Chebi:70708
19. 17-alpha-cyanomethyl-17-beta-hydroxy-estra-4,9(10)-dien-3-one
20. M 18575
21. M-18575
22. 2-((8s,13s,14s,17r)-17-hydroxy-13-methyl-3-oxo-2,3,6,7,8,11,12,13,14,15,16,17-dodecahydro-1h-cyclopenta[a]phenanthren-17-yl)acetonitrile
23. Zk-37659
24. Dienogest [inn]
25. Dienogestum [latin]
26. (17-hydroxy-3-oxoestra-4,9-dien-17beta-yl)acetonitrile
27. 17alpha-cyanomethyl-17beta-hydroxyestra-4,9(10)-dien-3-one
28. [(17beta)-17-hydroxy-3-oxoestra-4,9-dien-17-yl]acetonitrile
29. Unii-46m3ev8hhe
30. Dienogest [usan:inn:ban]
31. Endometrion (tn)
32. (17&alpha
33. Dienogest [jan]
34. 17-cyanomethyl-17-hydroxy-estra-4,9-dien-3-one
35. Dienogest [mi]
36. Dienogest [usan]
37. 17-alpha-cyanomethyl-17-beta-hydroxyestra-4,9(10)-diene-3-one
38. Dienogest [vandf]
39. 19-norpregna-4,9-diene-21-nitrile, 17-hydroxy-3-oxo-, (17alpha)-
40. Dienogest [mart.]
41. 17-hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile
42. Dienogest [who-dd]
43. Schembl37293
44. Dienogest (jan/usan/inn)
45. Gtpl7654
46. Sts557
47. Dienogest [orange Book]
48. Dienogest For System Suitability
49. Chembl1201864
50. Dienogest [ep Monograph]
51. Dienogest, >=98% (hplc)
52. Sh-t00660aa
53. Dtxsid80891478
54. 17-hydroxy-3-oxo-19-nor-17alpha-pregna-4,9-diene-21-nitrile
55. Bcp22681
56. Hy-b0084
57. Zinc4215629
58. Mfcd00868356
59. S1251
60. 19-norpregna-4,9-diene-21-nitrile, 17-hydroxy-3-oxo-, (17-alpha)-
61. Akos015840152
62. Akos015896680
63. Ac-2166
64. Ccg-267594
65. Cs-1782
66. Db09123
67. Ncgc00346502-04
68. D5230
69. D03799
70. Ab01566807_01
71. 928d587
72. Q139160
73. Sr-01000942230
74. Sr-01000942230-1
75. Brd-k50853363-001-02-3
76. Dienogest, Europepharmacopoeia (ep) Reference Standard
77. [(17?)-17-hydroxy-3-oxoestra-4,9-dien-17-yl]acetonitrile
78. 17-hydroxy-3-oxo-19-nor-17.alpha.-pregna-4,9-diene-21-nitrile
79. 19-norpregna-4,9-diene-21-nitrile, 17-hydroxy-3-oxo-, (17.alpha.)-
80. Dienogest For System Suitability, Europepharmacopoeia (ep) Reference Standard
81. 17-hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile, 17-cyanomethyl-17-hydroxy-estra-4,9-dien-3-one
| Molecular Weight | 311.4 g/mol |
|---|---|
| Molecular Formula | C20H25NO2 |
| XLogP3 | 1.8 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 1 |
| Exact Mass | 311.188529040 g/mol |
| Monoisotopic Mass | 311.188529040 g/mol |
| Topological Polar Surface Area | 61.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 679 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 4 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Indicated for use as the treatment of endometriosis alone and as a contraceptive in combination with ethinylestradiol.
Treatment of endometriosis
Dienogest exhibits a very potent progestagenic effect in the endometrium, and causes endometrial atrophy after prolonged use . It also mediates an antiandrogenic effect that is equivalent to approximately one third that of cyproterone acetate. A dose of 2 mg inhibits the growth of ovarian follicles at 10 mm and maintains the concentration of progesterone at a low level, but has a weak inhibitory effect on FSH and LH. 1mg/kg of dienogest also directly inhibits ovulation. In clinical trials composing of patients with endometriosis, dienogest therapy effectively reduced painful symptoms and endometriotic lesions associated with the disorder. Dienogest displays no antiestrogenic activity as it activate neither estrogen receptor (ER) nor ER, and causes hypoestrogenic effects instead as it is shown to decrease the relative expressions of ER and ER. It has no glucocorticoid or mineralocorticoid effects. In combined oral contraceptive pills (COCP) with ethinyloestradiol, dienogest conjuction therapy effectively reduces the symptoms of acne and hirsutism, as well as improving excessively heavy or prolonged menstrual bleeding.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Contraceptives, Oral, Hormonal
Oral contraceptives which owe their effectiveness to hormonal preparations. (See all compounds classified as Contraceptives, Oral, Hormonal.)
Contraceptive Agents, Male
Chemical substances or agents with contraceptive activity in males. Use for male contraceptive agents in general or for which there is no specific heading. (See all compounds classified as Contraceptive Agents, Male.)
Hormone Antagonists
Chemical substances which inhibit the function of the endocrine glands, the biosynthesis of their secreted hormones, or the action of hormones upon their specific sites. (See all compounds classified as Hormone Antagonists.)
G03DB08
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
G - Genito urinary system and sex hormones
G03 - Sex hormones and modulators of the genital system
G03D - Progestogens
G03DB - Pregnadien derivatives
G03DB08 - Dienogest
Absorption
Dienogest is rapidly absorbed following oral administration, with 91% bioavailability. The peak plasma concentration of 47 ng/mL is reached at about 1.5 hours after single ingestion of 2 mg. The stable concentrations of the drug are reached after two days of initial treatment.
Route of Elimination
The ratio of renal elimination to fecal elimination of dienogest is 3:1, where dienogest is predominantly excreted in the form of inactive metabolites. Most of orally administered drug is excreted in the urine within the first 24 hours of ingestion.
Volume of Distribution
The apparent volume of distribution (Vd/F) of dienogest is 40 L.
Clearance
The metabolic clearance rate from serum (Cl/F) is 64 mL/min.
Dienogest undergoes complete metabolism that is mainly mediated by CYP3A4. The metabolites are pharmacologically inactive and rapidly eliminated from the plasma.
Elimination half-life of dienogest is around 9-10 hours. The half-life of urinary metabolites excretion is 14 hours.
Dienogest acts as an agonist at the progesterone receptor (PR) with weak affinity that is comparable to that of progesterone but has a very potent progestagenic effect in the endometrium, causing endometrial atrophy after prolonged use. It promotes antiproliferative, immunologic and antiangiogenic effects on endometrial tissue. Dienogest reduces the level of endogenous production of oestradiol and thereby suppressing the trophic effects of oestradiol on both the eutopic and ectopic endometrium. Continous administration of dienogest results in hyperprogestogenic and moderately hypoestrogenic endocrine environment, which causes initial decidualization of endometrial tissue. It is an antagonist at androgen receptors, improve androgenic symptoms such as acne and hirsutism.


